Abstract
A retrospective study has indicated that stereotactic radiotherapy (SRT) has a value in treating both primary tumors and singular metastatic lesions that cause local symptoms. Here we present the results of a prospective study evaluating the safety and local efficacy of SRT in metastatic or inoperable primary renal cancer. Thirty patients with metastatic renal cell carcinoma (RCC) or inoperable primary RCC received high-dose fraction SRT. In total, 82 lesions were treated. Dose/fractionation schedules varied depending on target location and size. The most frequently used fractionations were 8 Gy×4, 10 Gy×4, 15 Gy×2 or 15 Gy×3 prescribed to the periphery of the PTV. Local control, defined as radiologically stable disease (SD) or partial/complete response (PR/CR) was obtained in 98% of treated lesions but 19% of lesions were in patients with a follow time of less than 6 months. CR was observed in 21% of the patients and 58% of the patients had a partial volume reduction or local stable disease after a median follow-up of 52 months (range 11–66) for patients alive and 18 months (range 4–57) for deceased patients. Local progression was seen in two lesions. Side effects were grade I–II in 90% of cases. The overall survival was 32 months. SRT for patients with primary and metastatic RCC resulted in high local control rate with generally low toxicity. The method can thus be considered a therapeutic option to surgery in patients with a limited number of metastases, as local treatment in RCC with an indolent presentation or as a method of reducing tumor burden prior to medical treatment.
About 60 000 persons are diagnosed with renal cell carcinoma (RCC) in the European Union every year Citation[1]. The reported incidence of RCC has increased by approximately 30% the past two decades, most likely reflecting both improved radiological diagnostic techniques and a true increase due to various environmental factors. Non-metastatic disease has high cure rates using surgery but in metastatic disease there are few therapeutic options and in most cases the disease progresses rapidly with a median survival time of 6 to 12 months and a 2-year survival rate of 10% to 20% Citation[2]. Renal cell cancer has however a varied biology and in a subset of patients, the disease presents in a more indolent form, progressing slowly over time and in rare cases, spontaneous temporary regression can be seen.
The most commonly used systemic therapy in RCC is interleukin-2 and interpheron-alpha but the response rates are low, ranging from 5–15% for both agents. Recently, new agents like for instance sorafenib Citation[3] and SU112484 Citation[4] have demonstrated impressive response figures installing hope of improved prognosis for patients with renal cancer. Selected patients with solitary or a limited number of distant metastases seem to achieve prolonged survival with nephrectomy and surgical resection of the metastases Citation[5], Citation[6]. Even selected patients with brain metastases may benefit from this treatment approach Citation[7].
Renal cell carcinoma is one of the more radioresistant tumor forms using conventional radiotherapy and this treatment has mainly been used as palliation of pain. In several clinical studies however, brain metastases treated with gamma-knife radiosurgery have yielded a local control rate of 90% and survival times similar to those achieved by surgery Citation[8], Citation[9]. Recently, the application of a similar radiation technique to extracranially located tumors has been made possible by the development of a stereotactic methodology for body targets enabling accurate localization of the tumor and optimal treatment with an accelerator Citation[10], Citation[11]. Previous experience with stereotactic high-dose fraction radiation therapy (SRT) in our department has demonstrated a high local control rate for a large number of tumor types (both primary tumors and metastases) located both above and below the diaphragm Citation[11–15]. SRT thus shows potential as a non-invasive alternative therapeutic approach to surgery when surgery is technically difficult or in patients with poor performance status with singular metastatic lesions.
This report accounts the results of a prospective phase II study to evaluate the local efficacy and safety of using extracranial SRT in RCC metastases and inoperable primary tumors.
Patients and methods
Patients
This open, single center prospective SRT study was approved by the local human investigations committee and a local ethics committee. The aim of the study was to evaluate efficacy and safety of SRT treatment. Local tumor response was used as primary endpoint and toxicity, pain and survival were secondary endpoints. Patients with inoperable primary, local recurrence or metastatic renal cell carcinoma with a life expectancy of more than 3 months were eligible for the study. No hormonal or immunotherapy was given within 4 weeks prior to treatment. No previous conventional radiotherapy to the tumor lesion chosen for SRT was accepted. Measurable lesions were required to be 10 mm or more in smallest dimension, or, if smaller than 10 mm, increasing in size at repeated CT examinations. Bone lesions were not accepted as targets but patients with bone metastases with other targets were eligible to the study. Patients with symptomatic brain metastasis or lesions located in proximity of organs where the risk of developing severe side effects was regarded as high were excluded. A minimal cycle dose of 8 Gy to the periphery of the PTV was a requirement. This dose should be possible to repeat at least three times in a week. All patients had a Karnovsky index of 60 or higher. Most patients included later in the study had not received any systemic treatments prior to SRT. In some instances, SRT was applied to new metastases that developed after the first SRT. Open biopsies or needle aspirations for histopathological examinations were obtained from patients with a long duration between primary surgery and development of recurrent disease (>2 years) and from patients with a single metastasis. At the time of treatment, all patients had one or more known lesions that had increased in size as assessed by repeated computer tomography scans.
The mean age at treatment was 64 years (range 43–79), 20 were men and 10 women. The total number of treated metastatic sites was 82. Of the 30 treated patients, five patients were treated for inoperable primary tumors whereof one had a singular metastatic lesion that was also treated; six patients were treated for singular metastases and 19 patients were treated for multiple metastatic lesions. The patients with metastatic disease had all been nephrectomized.
Six of 30 patients had received prior therapy and progressed before the first SRT-session (Two patients had interferon-α and two had prior Tamoxifen). Nine of 30 received general therapy after SRT due to progressive disease although none responded on this treatment (Five had interferon-α and four were treated with Tamoxifen or Medroxiprogesterone). No patients had adjuvant or concomittant treatment of any kind.
According to the prognostic criteria developed by Motzer et al. Citation[16] 50% of the patients had no risk criteria, 46.7% had 1 or 2 risk criteria and one patient had more than 2 risk criteria. lists the treated tumor locations. In this patient population, the first treatment was given in April 1999 and the last treatment in September 2004.
Table I. Treatment sites by organ. The majority of the treated lesions were located in the lungs. In some cases, the targets involved two organs explaining why the total number of treatment sites in the table exceeds 82.
Stereotactic radiotherapy (SRT)
The stereotactic methodology and the stereotactic frame (Elekta Oncology Systems) have been described in detail elsewhere Citation[10], Citation[11]. The technique enables accurate localization of the targets and geometrically accurate treatment with an accelerator. The patients are fixed within the frame by means of a vacuum pillow.
Indicators mounted inside the frame are visible on CT or MRI-scans, thus defining the stereotactic system. For set-up in the treatment room, there are scales mounted on the outside of the frame corresponding to the CT/MR indicators. To minimize diaphragm related motions to ±5 mm, an abdominal-pressure device was used. CT examinations were performed with the patient fixed in the stereotactic frame before dose planning. Shortly before the first treatment a second CT examination was done in order to study the reproducibility of positioning the target in the stereotactic system. The slice thickness of CT images was 5 mm. The reproducibility of repositioning the tumor in the stereotactic system has been demonstrated to be within a 5–8 mm in 90% of patients with liver and lung targets using this method Citation[10].
A clinical target volume (CTV) was defined on CT-scans. In most cases it was identical with the gross tumor volume (GTV). Around the CTV a margin of 5 or 10 mm was added in the transverse and 10 mm in the cranio-caudal directions to obtain the planning target volume (PTV). The margin in the transverse plane was 5 mm as a standard except for small lung targets, not connected to the pleura or mediastinum, in which the margin was 10 mm. These margins were based on our previous experience about the mobility of small lung tumors.
Treatment technique
The treatment used in the present study was a conformal technique using 5 to 8 coplanar or non-coplanar static beams of 6 MV, as previously described Citation[10], Citation[11]. The directions of the beams were spread in a large solid angle, taking into account the location of organs at risk. The beams were shaped by a multileaf collimator to conform to the target.
Dose distribution
The treatment technique used represents an isotropic convergent beam method with inhomogenous dose distribution and examples of the dose distributions and dose-volume histograms has been presented in detail previously Citation[10], Citation[17], Citation[18]. This principle was applied here by giving about a 50% higher dose to the target's central part, which may contain hypoxic cells, than the dose given at the periphery of the PT Citation[10], Citation[17], Citation[18]. The dose was prescribed at the periphery of the PTV Citation[10].
Three-dimensional dose-plans were made (TMS, Helax). Apart from dose distributions, calculated in a number of slices, dose-volume histograms were also calculated for the CTV, PTV and organs at risk. The pencil beam algorithm was used to calculate doses for all targets.
Geometrical verification
An important part in the methodology of SRT is to use CT for direct geometrical verification of the target position in the stereotactic system, instead of indirect verification of bony landmarks by portal imaging Citation[9]. Thus, a new CT examination was done shortly before the first treatment to verify reproducibility. The dose planning system was also used to compare different CT examinations to determine the reproducibility of the target position in the stereotactic system Citation[11].
Fractionation
Treatments were given in 2 to 5 fractions, with 5–15 Gy per fraction prescribed to the periphery of the PTV. The treatments were generally given every second day. Several factors were taken into consideration when deciding the dose/fraction, number of fractions and time between fractions. These included size of the CTV and dose distribution to surrounding radiation sensitive normal tissues. In one case total doses below 8 Gy×3 were delivered in a secondary session due to closeness of the target to risk-organs.
shows the fractionation schemes used. gives the total mean dose to CTV as EQD2 (α/β = 5) versus the CTV volume. (EQD2) was calculated for an α/β equal to 5, which is a reasonable estimate of the value for renal cell carcinoma Citation[15].
Figure 1. The total mean dose to the CTV and the CTV volume is displayed for all targets. The dose is expressed as EDQ2 for α/β = 5. Circles represent targets with local progression.
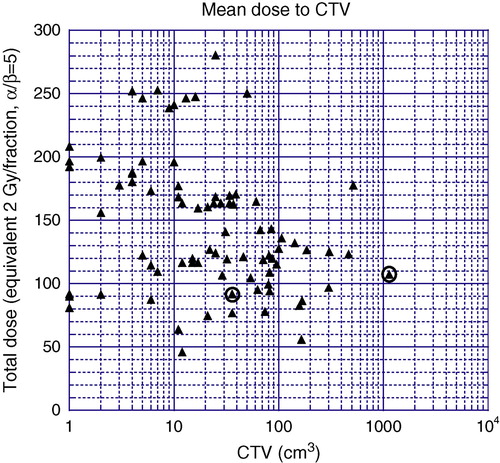
Table II. Fractionations used. The fractionation schedule 5 Gy×5 was used in a target close to a risk organ at a secondary treatment occasion.
The mean value of the mean doses to CTV was 1.40±0.13 (SD) times the mean value of the prescribed doses.
No single fixed dose recommendation or fractionation covers all target sizes and all risk organs. The fractionation was not prescribed until an optimal dose plan could be examined and dose-volume histograms of target volumes and organs at risk were evaluated. General guidelines concerning dose/fractionation recommendations have been described elsewhere Citation[15].
Follow-up after treatment and definition of responses
Clinical examination and CT-scans were performed at 3 month intervals following SRT. Intervals between clinical examinations were extended to 6 months in patients without recurrences or stable disease for more than 2 years. Progression was defined as more than 120% of the pre-treatment value, stable disease as 50–120% of pre-treatment value and regression as <50% of pre-treatment value and total regression as tumor no longer visible on CT-scans. Traditional radiological response criteria may be suboptimal when evaluating local response to radiotherapy since conventional radiological techniques cannot differentiate between viable and non-viable tissue. It is well documented that radiotherapy in many instances result in local scar tissue formation and SD may thus in many cases represent a CR. Nine patients underwent positron emission tomography (PET) in order to determine this methods additional value compared to CT-scans in evaluating responses and for differentiating viable and non-viable tumor tissue.
The CALGB expanded common toxicity criteria (NCI) were used for evaluation of toxicity.
Results
The overall median follow-up time was 52 months (SD 19, range 11–66 months) for patients alive and 22 months for deceased patients (SD 16, range 4–57). Twenty one patients were treated with SRT once, six were treated twice, one patient three times and two patients on four occasions. In 21% of the SRT-treated metastatic sites, total tumor regression (CR) was seen after 3 to 36 months, 31% treated sites showed regression of more than 50% after 3 to 12 months and 27% responded without a significant volume reduction. In two lesions (2%) local progression was seen. Nineteen percent of the lesions had a follow-up time of less than 6 months either because of early death (n = 6), or unwillingness to undergo follow-up examinations (n = 1). Among these patients, there was no sign of local progression in the treated lesions. Local control rate was thus 79% if all patients with shorter than 6 months follow-up are regarded as possible failures and 98% if they are regarded as local responders. Currently, 11 patients are alive and 19 have died.
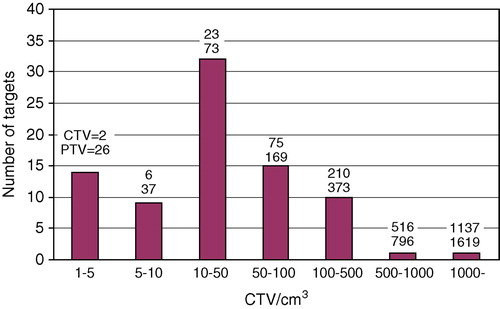
In the number of targets is shown within different CTV volume intervals. The upper figure above each column gives the mean value of the CTV (cm3) for each group of targets, and the lower figure the mean of the PTV volumes.
Figure 2. The number of targets are shown within different CTV volume intervals. The upper figure above each column gives the mean value of the CTV (cm3) for each group of targets, and the lower figure the mean of the PTV volumes.
Local progressive cases
Case 1
CTV included kidney loge and part of the liver with diffuse tumor growth. CTV was 1 136 cm3 (right-most circle in ) and the prescribed dose was 8 Gy×5. The very large CTV together with diffuse growth and the relatively low dose are several factors that, added together, are a very reasonable explanation for the local progression, seen after 11 months.
Case 2
CTV included the primary tumor in the remaining kidney. The CTV was 36 cm3 (left-most circle in ) and the prescribed dose 10 Gy×3. Due to the vicinity to the stomach and bowels, the dose planning was made in a way that resulted in that both the anterior and cranial part of the PTV was underdosed. This may be a likely explanation for the progression, seen after 54 months.
Both of these local progressive cases were retreated (8 Gy×5 and 10 Gy×4) and are still alive without disease after 3 years and 8 months FU.
PET-scans indicated regression in three of nine patients (33%) after 3 months and regression in six of nine patients after 6 months.
Side effects of SRT
The most common side-effects were cough, fatigue, skin rash and local pain. Side effects were seen in 16 of 28 patients and 96% of registered side effects were grade I–II. One patient treated for a large metastatic lesion (516 cm2) in the lung close to the pleura died 10 weeks after SRT treatment (12 Gy×4) after being admitted to hospital with electromechanical dissociation. No autopsy was made but it cannot be ruled out that the fatality may have been treatment related.
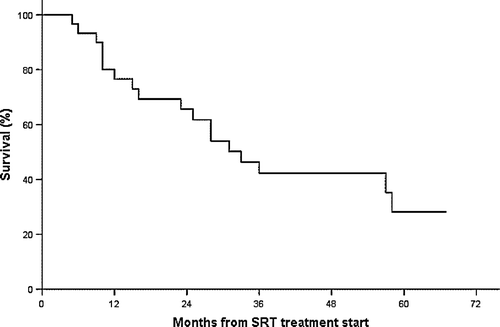
Survival
The overall median survival was 32 months. Currently, 11 are alive and 19 have died. A Kaplan-Meier plot for survival is shown in .
Discussion
The rationale for applying SRT technique in RCC in extracranial metastatic lesions was the former good results using gamma-knife radiosurgery for brain metastases in RCC Citation[9] and data indicating that selected patients benefit from surgical resection of singular metastases.
Previously published retrospective analysis of SRT treatment in RCC has indicated that the methodology and dose fractionation used in our center achieves at least 90% local tumor control and the registered side effects have been mild Citation[15]. This prospective study corroborates these findings with a local control rate in 98% of the treatments and generally low toxicity. The results also demonstrate that RCC is not necessarily radioresistant but sensitive to higher dose fractions.
In the present material the two targets with local treatment failure are illustrated by circles in . For a successful treatment in terms of local control, there is an upper limit of the target volume and a lower limit of the target dose that have to be respected. With only two local failures in this material further data is needed for a definite conclusion.
When it comes to risk organs and tolerance for these hypofractionated schedules there must be an individual modification of the total dose and fractionation with regards to the volume of the risk organ included in the CTV/PTV or its immediate proximity and an estimation of the possible movements of risk organ-volumes into the radiation field. Here the choice between equipotential doses 12 Gy×2 or 8 Gy×4 would favour the latter one since this fractionation is less toxic for normal tissue (α/β = 10).
The diverse biology of RCC makes patient selection difficult. Patients where the disease presents in a more indolent form where the time between primary surgery and first metastases is more than 12 months and patients with few metastatic lesions are most suitable for SRT whereas using the technique in patients with a rapidly progressing disease is meaningful only when there are focal symptoms due to local metastases or as method of reducing bulky disease prior to antitumoral drug or immunotherapy.
There is data indicating that high dose radiotherapy sometimes triggers regression of untreated metastases. There are currently evidence supporting that this effect may be due to either radiation induced immune response or the production of angiogenesis inhibiting substance/s Citation[19]. In our previous material of 27 patients with evaluable non-treated lesions, systemic responses were seen in 14% of patients Citation[20]. In the present study, no regressions of untreated metastases were seen but in the majority of patients all known metastatic lesions were treated and thus possible antitumoral effect on micrometastases cannot be excluded. The median survival is however remarkably high in this material, but this may partly be explained by patient selection. The average number of Motzer prognostic criteria Citation[16] in our group of patient was low and thus a longer survival was expected.
In summary, we have established in a Phase II trial that using SRT for patients with primary and metastatic RCC provides a high degree of local control rate (98%). Toxicity was low but great care needs to be taken with regards to organs at risk and when treating larger lesions.
References
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; 16: 481–8
- Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol 2003; 4: 385–90
- Escudier B, Szczylik C, Eisen T, Ondard S, Staehler M, Negrier S, et al. Randomized phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). J Clin Oncol (Meeting Abstracts) Late Breaking Abstracts 2005;23: 1093s. Abstracts 4510.
- Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sutinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295(21)2516–24
- Cerfolio RJ, Allen MS, Deschamps C, Daly RC, Wallrichs SL, Trastek VF, et al. Pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg 1994; 57: 339–44
- Fourquier P, Regnard JF, Rea S, Levi Fand Leuasseur P, et al. Lung metastases of renal cell carcinoma: results of surgical resection. Eur J Cardiothoracic Surg 1997; 11: 17–21
- Wronski M, Arbit E, Russo P, Galicich JH. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology 1996; 47: 187–93
- Karlsson B, Wersäll P, Lippitz B, Kihlström L. Repeated radiosurgery versus fractionated radiotherapy in the treatment of brain metastases from renal cell car. Radiosurgery, D Kondziolka. Basel, Karger 1999; 232–9
- Kihlstrom L, Karlsson B, Lindquist C, Noren G, Rahn T. Gamma knife surgery for cerebral metastasis. Acta Neurochir Suppl (Wien) 1991; 52: 87–98
- Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Lax I, Blomgren H, Larson D, Näslund I. Extracranial stereotactic radiosurgery of localized targets. J Radiosurgery 1998; 1: 135–48
- Gunven P, Blomgren H, Lax I. Radiosurgery for recurring liver metastases after hepatectomy. Hepatogastroenterology 2003; 50: 1201–4
- Schefter T, Gaspar LE, Kavanagh B, Ceronsky N, Feiner A, Stuhr K, et al. Hypofractionated extracranial stereotactic radiotherapy for liver tumours. Int J Radiat Oncol Biol Phys 2003; 57(Suppl 2)S282
- Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: A noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004; 60: 186–96
- Wersall PJ, Blomgren H, Lax I, Kalkner KM, Linder C, Lundell G, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005;77:88–95. Epub 2005 Jun 20.
- Schwartz LH, Mazumdar M, Wang L, Smith A, Marion S, Panicek DM, et al. Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer 2003; 98: 1611–9
- Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumours using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Lax I. Target dose versus extratarget dose in stereotactic radiosurgery. Acta Oncol 1993; 32: 453–7
- Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res 2003; 63(8)1990–3
- Wersäll P, Blomgren, H, Lax I, Pisa P, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Accepted for publication Acta Oncol 2006.