Abstract
Seventy patients with advanced head and neck cancer were treated with vinorelbine and continuous 5-FU administered in a central venous catheter. Over all response was 36% with 9% complete responses. The most common grade 3 and 4 toxicities were stomatitis (13), infection (5), pain related to vinorelbine infusion (4), skin toxicity (3). Thirty one patients had grade 3 or 4 leukopenia. Treatment was complicated by venous thrombosis in the central venous catheter in one case. A majority of patients experienced dose reduction of one or both drugs or treatment delays due to toxicity. Median time to progression was 4.7 months and overall median survival 6.6 months. We conclude that the regimen is feasible and tolerated with moderate toxicity. Response rates and time to progression are comparable to other studies with multi agent treatment.
Metastatic or recurrent head and neck cancer not amenable to radiotherapy or surgery has a poor prognosis with a median survival time of approximately 4 months Citation[1] without treatment. The disease has great functional and cosmetic implications for the patient.
There is no standard treatment for patients with recurrent or metastatic head and neck cancer. Studies investigating chemotherapy versus best supportive care have demonstrated a 10 week survival advantage Citation[2]. Single agent treatments show response rates of 10–30% in phase II studies Citation[3], Citation[4] with the highest response rates seen with cisplatinum and taxanes Citation[5–7]. Combination chemotherapy have shown response rates of 30–60% Citation[5], Citation[8–14]. Response rates tend to be about 30–35% in randomised phase III trials Citation[4], Citation[15–17]. The highest response rates are seen in combinations including cisplatinum Citation[9], Citation[18]. It has not, however, been possible to demonstrate that higher response rates improves overall survival Citation[4], Citation[15], Citation[19], Citation[20]
Previously the combination of cisplatinum and 5-FU Citation[21] and more recently the combination of platinum and taxanes has been considered “standard of care”. These regimens are known to exhibit moderate to high toxicity and are often given in an in patient setting.
Since the aim of chemotherapy in advanced head and neck cancer is mainly palliation the toxicity of the treatment should not exceed the advantages of potential symptom relief by chemotherapy.
Both vinorelbine and continuous intravenous infusion of 5-FU have shown single agent response rates of 10–20% Citation[22–25]. Both drugs show low to moderate toxicity Citation[22] and 5-FU has less pronounced toxicity and potentially higher efficacy when administered continuously Citation[26]. Both drugs can be administered in an out patient setting.
This study was performed in order to investigate the feasibility of a regimen in which toxicity was expected to be low to moderate and at the same time assuring that response rates and survival were comparable to other combination regimens. Furthermore we wanted to evaluate a regimen that could potentially be used in combination with radiotherapy for advanced head and neck cancer with curative intent.
Patients and methods
Patients from three Danish departments of oncology were enrolled in this phase II study between 1998 and 2003. The following inclusion criteria had to be met: 1) metastatic or recurrent squamous cell carcinoma in the head and neck not amenable for surgery or radiotherapy, 2) WHO performance status ≤2, 3) adequate liver, renal and bone marrow function, 4) evaluable disease according to WHO criteria, 5) oral and written informed consent.
The study was approved by the Ethics Committees and the Danish Medicines Agency.
Chemotherapy consisted of vinorelbine and 5-FU. Vinorelbine was given as a 10 min infusion intravenously at a dose of 25 mg/m2 once a week. 5-FU was administered through a central venous catheter (CVC) at a daily dose of 300 mg/m2 /day given continuously delivered by an electronic portable infusion pump, which was refilled weekly, the same day as the vinorelbine infusion was given. Treatment was continued for 26 weeks or until progression or unacceptable toxicity as determined by either the patient or the physician. A treatment cycle consisted of one week of treatment. Patients were considered to have entered a treatment cycle if day one treatment was given.
A blood count was performed each week. If the WBC was < 2.0×10 9/l or platelets were < 75×10 9/l vinorelbine was delayed until restitution and reduced by 20% in subsequent courses.
5-FU was delayed if WBC was < 1.0×109/l or platelets were < 50×109/l and after restitution continued with 20% dose reduction. In case of grade III or IV stomatitis, dermatitis and diarrhoea 5-FU infusion was delayed until full restitution and then continued with 20% dose reduction.
Patients were evaluated for efficacy every 8 weeks and for toxicity every 4 weeks according to WHO criteria Citation[27]
Primary endpoints were response and time to progression. Secondary endpoints were overall survival, toxicity and feasibility of the regimen.
Time to progression was calculated from the first day of treatment to the date of progression or the date of death from disease. Over all survival was calculated from the first day of treatment to the date of death. Kaplan-Meier statistics were used to evaluate time related endpoints. Data were analysed in the statistics program Medlog (Window edition).
Results
Seventy patients were enrolled in the study and patients received at least one course of chemotherapy. Patient characteristics are shown in .
Table I. Patient characteristics
The median number of treatment cycles was 14 (range 1–30). Dose reduction of vinorelbine was performed in 31 patients (44%) and 46 patients (66%) had one or more vinorelbine courses delayed. 5-FU dose was reduced in 40 patients (57%) and 37 (53%) had one or more courses delayed. Forty three patients (61%) had some kind of dose modification in both agents during treatment time, that being either delay of chemotherapy or dose reduction. The most common reason for dose modification of vinorelbine was leukopenia. Stomatitis was the most common reason for dose modification of 5-FU.
Haematological toxicity
Of 70 patients one patient did only receive one course of chemotherapy and did not return for the planned blood tests. Thirty one patients (44%) experienced grade 3 or 4 leukopenia and no patients had grade 3 or 4 thrombocytopenia.
Non haematological toxicity
Three patients received less than three courses and did not return for toxicity registration. Grade three and four toxicities included stomatitis in 13 (18%) patients, infection in five (7%) patients, pain during vinorelbine infusion in four (6%) patients, hand-foot syndrome in three (4%) patients and diarrhoea in two. Nausea, constipation and fatigue were seen in one patient. There was one toxic death due to infection after placing a feeding tube.
Complications with the central venous catheter were observed in 12 (17%) patients. Five patients had an infection related to the central venous catheter. In five cases it accidentally fell out and in one patient the catheter was replaced because of malfunctioning. Only one patient had a venous thrombosis with possible relation to the catheter.
Among 70 patients 6 (9%) were not evaluable (NE) for response. One patient had renal cell carcinoma diagnosed during chemotherapy and was taken off the study without being evaluated. One patient stopped treatment due to toxicity and was offered chemotherapy with gemcitabine before evaluation. In one patient treatment was stopped after 2 days because the patient's WHO performance status was reconsidered. Two patients who received one and six courses of chemotherapy respectively were not evaluated as planned. The patient who died from toxicity was not evaluated for response. Over all response rate was based on “intention to treat” and was 36%. Six (9%) patients achieved complete response and 19 (27%) partial response.
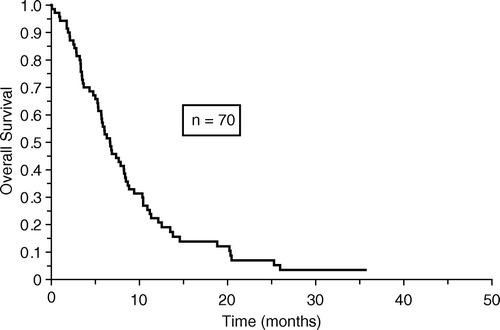
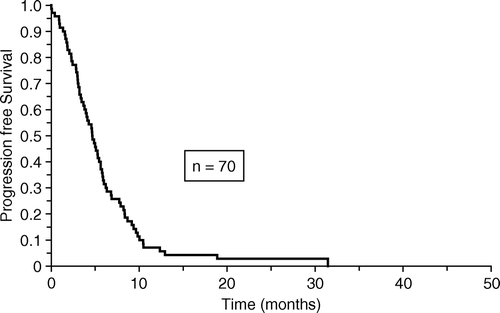
No patients were lost from follow-up for survival. Overall median survival for the 70 patients was 6.6 months and median time to progression was 4.7 months as shown in and , respectively.
Discussion
The aim of this study was to investigate a regimen that was potentially less toxic than the standard treatment with cisplatin and 5-FU and that could be given in an out patient setting. Another objective of this study was to evaluate a treatment regimen that could potentially be used in a setting of concomitant chemoradiotherapy for advanced head and neck cancer.
In this study we found a median survival of 6.6 months which is comparable to the results of other studies using combination chemotherapy Citation[11], Citation[14]. The median time to progression of 4.7 months is also comparable to other studies Citation[14].
Although our response rates were similar to those in other studies Citation[10], Citation[11], Citation[13], Citation[14] we had expected that the toxicity profile to be more favourable, but especially leukopenia and infections were more pronounced than expected. Leukopenia was the most common reason for reducing dose of vinorelbine. The treatment is manageable as in an out patient setting and the central venous catheter was not a major problem. Some of the infections with possible relation to the catheter could perhaps have been avoided if leukopenia had been less pronounced.
Stomatitis was the most common reason for reducing dose of 5-FU. The rate of stomatitis and skin toxicity gives some considerations in using this regimen in concomitant radiotherapy for advanced head and neck cancer. Oral mucositis is a dose limiting toxicity in treating head and neck cancer with radiotherapy. The regimen does not have better response rates or a more favourable toxicity profile compared to other regimens used for recurrent or metastatic head and neck cancer.
References
- Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer 2000; 36: 1032–7
- Morton RP, Rugman F, Dorman EB, Stoney PJ, Wilson JA, McCormick M, et al. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: A randomised factorial phase III controlled trial. Cancer Chemother Pharmacol 1985; 15: 283–9
- Pinto HA, Jacobs C. Chemotherapy for recurrent and metastatic head and neck cancer. Hematol Oncol Clin North Am 1991; 5: 667–86
- Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 1992; 10: 257–63
- Guaraldi M, Martoni A, Tononi A, Occhiuzzi L, Caliceti U, Dall‘Olia D, et al. 5-Fluorouracil + folinic acid with cisplatinum and bleomycin in the treatment of advanced head and neck squamous cell carcinoma. Ann Oncol 1991; 2: 379–81
- Forastiere AA, Shank D, Neuberg D, Taylor IV SG, DeConti RC, Adams G. Final report of a phase II evaluation of paclitaxel in patients with advanced squamous cell carcinoma of the head and neck. An Eastern Cooperative Oncology Group Trial (PA390). Cancer 1998; 82: 2270–4
- Dreyfuss AI, Clark JR, Norris CM, Rossi RM, Lucarni JW, Busse PM, et al. Docetaxel: An active drug for squamous cell carcinoma of the head and neck. J Clin Oncol 1996; 14: 1672–8
- Hussain M, Gadgeel S, Kucuk O, Du W, Salwen W, Ensly J. Paclitaxel, Cisplatin, and 5-fluorouracil for patients with advanced or recurrent squamous cell carcinoma of the head and neck. Cancer 1999; 89: 2364–9
- Shin DM, Glisson BS, Khuri FR, Ginsberg L, Papadimitrakopoulou V, Lee JJ, et al. Phase II Trial of paclitaxel, ifosfamide, and cisplatin in patients with recurrent head and neck squamous cell carcinoma. J Clin Oncol 1998; 16: 1325–30
- Okuno SH, Mailliard JA, Suman VJ, Edmonson JH, Creagan ET, Nair S, et al. Phase II study of methotrexate, vinblastine, doxorubicin, and cisplatin in patients with squamous cell carcinoma of the upper respiratory or alimentary passages of the head and neck. Cancer 2002; 94: 2224–31
- Espinosa, E, Zamora, P, Millá, A, Morales, S, Molina, R, Mira, M, et al. A phase II trial of cisplatin and vinorelbine in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck 2002;12:1054–9.
- Gedlicka C, Formanek M, Selzer E, Burian M, Kornfehl J, Fiebiger W, et al. Phase II study with docetaxel and cisplatin in the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck. Oncology 2002; 63: 145–50
- Clark JI, Hofmeister C, Choudhury A, Matz G, Collins S, Bastian R, et al. Phase II evaluation of paclitaxel in combination with carboplatin in advanced head and neck carcinoma. Cancer 2001; 92: 2334–40
- Hitt R, Castellano D, Hidalgo M, Garcia-Carbonero R, Pena M, Brandariz A, et al. Phase II trial of cisplatin and gemcitabine in advanced squamous-cell carcinoma of the head and neck. Ann Oncol 1998; 9: 1347–9
- Clavel M, Vermorken JB, Cognetti F, Cappelaere P, de Mulder PHM, Schornagel JH, et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 1994; 5: 521–6
- Forastiere AA, Leong T, Rowinsky E, Murphy BA, Vlock DR, DeConti RC, et al. Phase III comparison of high-dose paclitaxel + cisplatin + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer. J Clin Oncol 2001; 19: 1088–95
- Schrijvers D, Johnson J, Jiminez U, Gore M, Kosmidis P, Szpirglas H, et al. Phase III trial of modulation of cisplatin/fluorouracil chemotherapy by interferon alfa-2b in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 1998; 16: 1054–9
- Shin DM, Glisson BS, Khuri FR, Ginsberg L, Lawhorn K, Waun KH, et al. Recent advances in paclitaxel-containing chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck. Semin Oncol 1997; 24: 33–7
- Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: A Southwest Oncology Group Study. J Clin Oncol 1992; 10: 1245–51
- The Liverpool Head and Neck Oncology Group. A phase III randomised trial of cistplatinum, methotrexate, cisplatinum + methotrexate and cisplatinum + 5-FU in end stage squamous carcinoma of the head and neck. Br J Cancer 1990; 61: 311–5
- Browman GP, Cronin L. Standard chemotherapy in squamous cell head and neck cancer: What we have learned from randomized trials. Semin Oncol 1994; 21: 311–9
- Sørensen P, Andersen LJ, Hansen O, Bastholt L. Long-term continuous 5-fluorouracil infusion in patients with advanced head and neck cancer. Acta Oncol 1999; 38: 1043–5
- Tapazoglou E, Kish J, Ensley J, Al-Sarraf M. The activity of a single-agent 5-fluorouracil infusion in advanced and recurrent head and neck cancer. Cancer 1986; 57: 1105–9
- Gebbia V, Testa A, Valenza R, Zerillo G, Restivo S, Ingria F, et al. A pilot study of vinorelbine on a weekly schedule in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 1993; 29A(9)1358–9
- Testolin A, Recher G, Cristoferi V, Gasparini G. Vinorelbine in pre-treated advanced head & neck squamous cell carcinoma. A phase II study. Invest New Drugs 1994; 12: 231–4
- Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program Study. J Clin Oncol 1989; 7: 425–32
- WHO. Handbook for Reporting Results of Cancer Treatment. World Health Organization, Geneva 1979