Abstract
Vascular endothelial growth factor-C (VEGF-C) is involved in lymphatic metastatic spread. Metastatic site is a prognostic factor in melanoma. We assessed whether serum levels of VEGF-C are associated with metastatic sites or prognosis in patients treated for stage IV melanoma. The study included 64 patients, who received dacarbazine or four-drug chemotherapy (dacarbazine, vincristine, bleomycin and lomustine; BOLD) both combined with interferon-alfa. Serum samples for VEGF-C were analyzed by ELISA. The patients (n =22) with only skin and subcutaneous metastases had significantly lower mean VEGF-C levels (1 643 pg/ml) then the patients (n =42) with other distant metastases (2 584 pg/ml, Mann-Whitney, p =0.033). VEGF-C levels above the median (1 590 pg/ml) were significantly related to deep lymph node involvement (OR 3.763; 95% CI 1.038 − 13.646, p =0.034). There were no other significant associations between VEGF-C levels and tumour burden, nor were the levels significantly related to the response to therapy or survival. Those eight patients, who had received previous adjuvant IFN-alfa therapy had lower mean VEGF-C levels (1 738 pg/ml) as compared to those 56 patients without previous IFN-alfa therapy (2 335 pg/ml, ANOVA, p =0.026). This is the first study exploring serum VEGF-C in melanoma. VEGF-C might be involved in the deep lymphatic dissemination and progression of melanoma metastasis.
VEGF-C belongs to a family of vascular endothelial growth factors (VEGFs). This family is composed of angiogenic growth factors VEGF-A, VEGF-B, VEGF-C and VEGF-D and their corresponding receptor tyrosine kinases VEGFR1 (Flt-1), VEGFR-2 (Flk-1, KDR), and VEGFR-3 (Flt-4) Citation[1]. VEGFs regulate multiple angiogenic and lymphangiogenic processes and their role as prognostic factors in different human tumours has been well established. VEGF-C and VEGF-D have some similarity in their function as lymphangiogenic factors, but their expression and function profiles vary in different tumour types. Recent studies suggest that the mechanisms by which VEGF-C promotes lymph node metastasis are different from those of VEGF-D Citation[2]. Interestingly, the function of peri-tumoural lymphatic vessels induced by VEGF-C have exhibited abnormal functions, e.g. retrograde draining pattern Citation[3]. In epithelial ovarian carcinoma, both VEGF-C and –D were elevated and indicated lymphatic spread and intra-peritoneal dissemination, but only VEGF-D was an independent predictor of poor survival Citation[4].
VEGF-C shows particular activity in lymphangiogenesis and lymphatic spread of tumour cells, as shown in patients with gynecological cancers Citation[5–7], lung cancer Citation[8], Citation[9], gastrointestinal tract malignancies Citation[10], Citation[11] and breast carcinoma Citation[12]. Most of the recent studies have measured this factor in tumour samples, where its increased levels usually correlate with poor prognosis and disease recurrence. There are only few studies on serum VEGF-C measurements. Tamura et al. showed that serum VEGF-C levels were associated with lymphatic vessel invasion and development of lymph node metastases in non-small cell lung carcinoma (NSCLC) patients Citation[13]. Similarly, circulating MMP-9 and VEGF-C levels correlated with lymph node metastasis in these patients Citation[14]. Recently, Mathur et al. Citation[15] and Mitsuhashi et al. Citation[16] reported that high serum VEGF-C levels are associated with disease recurrence or persistence in patients with cervical carcinoma.
Recent studies on melanomas have shown that VEGF-C expression in the tumour samples correlates with disease progression Citation[17]. Similarly, tumour VEGF-C expression correlates with dissemination of melanoma metastases into the lymph nodes Citation[18]. These anecdotal observations prompted us to assess, whether serum levels of VEGF-C might be associated to a) key clinical variables, b) spread of metastases at distinct body sites, c) treatment response, or d) progression-free and overall survival in patients receiving chemo-immunotherapy for advanced melanoma.
Materials and methods
Patients
The material of this study is a sub-series of a previous study, in which 108 patients with advanced melanoma were enrolled into a Finnish randomized multi-centre trial, conducted in five University Hospitals between years 1995 and 2001. The details of the trial have been reported recently Citation[19]. The treatment arms were: A) dacarbazine plus natural IFN-α, B) a four-drug chemotherapy composed of dacarbazine, vincristine, bleomycin and lomustine (BOLD) plus natural IFN-α, C) dacarbazine plus recombinant IFN-α2b and D) BOLD plus recombinant IFN-α2b. The current series included 64 patients, of which 39 were males and 25 were females, with the median age of 62 years (range 30–75). All patients enrolled in this study had a progressive, inoperable, metastatic melanoma Citation[19]. Some patients had previously detected metastases, and others entered the study after the appearance of their first metastasis. Serum VEGF-C was analyzed in all patients samples, which were available for analysis. There was no any patient selection between centers in this sub-series.
The key characteristics of the patients are summarized in (). All serum samples evaluated here for VEGF-C were collected before starting the chemo-immunotherapy.
Table I. Key characteristics of the patients.
Approval by the local Ethical Committees and written informed consent were obtained before the initiation of the original study and the current analysis.
Serum VEGF-C detection
Quantitative analysis of serum VEGF-C was performed by using a commercial ELISA enzyme immunoassay, performed following the manufacturer's instructions (IBL Immuno-Biological Laboratories Co., LTD, Gunma, Japan). This VEGF-C assay determines total VEGF-C in serum. Briefly, serum samples were incubated in a micro-well plate pre-coated with anti-human VEGF-C rabbit IgG antibody. Any VEGF-C present in the samples was bound to the wells, and the excess was removed by extensive washing. The amount of VEGF-C was detected by a peroxidase-labelled antibody, and the amount of peroxidase was determined by addition of tetramethylbenzidine (TMB) substrate. Reactions were stopped by adding an acid solution, and the optical density was read at 450 nm in a micro-titer plate spectrophotometer. Serum concentrations were determined from the corresponding standard curves run for each plate separately. All measurements in these pre-treatment samples were done as duplicates and the mean value was chosen to represent the VEGF-C value of each patient.
Statistical analysis
Statistical analyses were performed using the SPSS® (SPSS, Inc., Chicago, USA) and STATA (Stata Corp., Texas, USA) software packages (SPSS for Windows, version 13.1.and STATA/SE 9.1). Frequency tables were analysed using the χ2 test, with likelihood ratio (LR) or Fischer's exact test being used to assess the significance levels between the categorical variables. Odds Ratios (OR) and their 95% Confidence Intervals (95% CI) were calculated where appropriate, using the exact method. Differences in the means of continuous variables were analysed using the non-parametric tests (Mann-Whitney, Kruskal-Wallis) or ANOVA (analysis of variance), after rigorous testing for the normal distribution by the Kolmogorov-Smirnov test of the log-transformed VEGF-C values. Univariate survival (life-table) analysis for the outcome measure (overall survival, progression-free survival, treatment-associated survival) was based on Kaplan-Meier method. In all tests, the values p < 0.05 were regarded statistically significant.
Results
Serum levels of VEGF-C in patient samples
The median serum level VEGF-C in the whole series was 1 590 pg/ml (89–7102 pg/ml) and the mean level was 2 260 pg/ml. Patients′ sex or age did not have a significant impact on the mean levels, although the levels were slightly higher (2 371 pg/ml) in women that in men (2 189 pg/ml). The site of the primary tumour was not significantly associated to serum VEGF-C levels (Kruskal-Wallis, p = 0.297), although the patients with the primary tumour in the trunk or mucosal sites had somewhat higher VEGF-C levels (). The difference between the trunk and upper limb was significant (p = 0.021 ANOVA, LSD). Serum VEGF-C levels did not correlate with the histological type of the primary tumour or its Breslow or Clark's classification (data not shown). Serum VEGF-C levels were not significantly associated with tumor burden. In patients with one metastatic site only the mean serum VEGF-C levels were 1 970 ng/ml and in those with several metastatic sites 2 800 ng/ml (p = 0.065).
Figure 1. Serum VEGF-C levels related to the site of the primary tumour. NUD – non ultra descriptus (unknown primary site). The amount of the analysed mucosal melanomas is two.
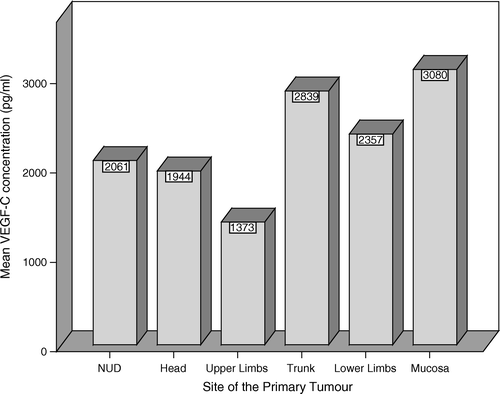
Serum VEGF-C variation was large (89–7 102 pg/ml) between the patients with the lowest and the highest value. However, no marked differences in patient characteristics between these two patients were detected. The patient with the lowest serum VEGF-C value had her primary tumour in lower limb and first metastases in inguinal lymph nodes. After surgery she had had previous adjuvant IFN-α therapy before entering the metastatic phase. Metastases were detected in liver and skin before entering the study. The best response for therapy was PD and TTP was three months.
The patient with the highest serum VEGF-C value had also her primary tumour in lower limb and the first metastases in inguinal lymph nodes. After surgery and radiation therapy she had no adjuvant therapy. Skin, liver and deep lymph node metastases were detected before entering the study. The best treatment response was PD and TTP was 2.1 months.
VEGF-C levels and skin and subcutaneous metastases
In patients with only skin or subcutaneous metastases (n = 22), the mean VEGF-C level was 1 643 pg/ml as compared with 2 584 pg/ml in the patients with other metastatic deposits (n = 42, Mann-Whitney U test, p = 0.033) ().
Table II. Serum VEGF-C levels and metastatic sites.
VEGF-C levels and involvement of deep lymph nodes
Altogether, almost 75% (11/15) of the patients with deep lymph nodes involved had VEGF-C levels above the median (1 590 pg/ml), in contrast to 58% (26/45) of those without deep lymph node involvement, who had their VEGF-C levels below the median (). Thus, the patients with higher VEGF-C levels had an increased risk of having deep nodal involvement (OR = 3.763; 95% CI 1.038–13.646, p = 0.034).
VEGF-C levels and other metastatic sites
Of the 64 patients, 33 had lung metastases with or without simultaneous soft tissue involvement (). In these patients, the mean serum VEGF-C level was 2 024 pg/ml. Of the patients with lung metastases, 39% had VEGF-C levels above median and 61% had values below. The patients with liver metastases had also slightly higher VEGF-C concentrations (mean 2 436 pg/ml) than those without liver metastasis (mean 2 132 pg/ml)(N.S.). Median VEGF-C level was not prognostic for liver metastasis, while 48% of those patients had values above median and 52% below it.
VEGF-C and treatment response
We also tested, whether serum VEGF-C levels were associated with a) overall survival (OSS, time from the primary diagnosis until death), b) time to progression (=PFS, progression-free survival) or c) survival after initiation of the chemoimmunotherapy (=TAS, treatment-associated survival). The results were disappointing, since no significant relationships were disclosed. However, in those eight patients, who had received adjuvant IFN-α therapy before the start of treatment for their metastatic disease, serum VEGF-C levels were significantly lower (mean 1 738 pg/ml) as compared to those (mean 2 335 pg/ml) of the other 56 patients, who did not receive any previous adjuvant IFN-α therapy (ANOVA, p = 0.026). By chance, all those eight patients with previous adjuvant IFN-α therapy were randomized to the BOLD & IFN-α treatment arm (), and thus, the “risk” of having VEGF-C levels below median in this treatment arm had OR = 4.20 (95% CI 1.47–11.93) (p = 0.005).
Table III. VEGF-C levels of the patients in different treatment arms.
Discussion
Melanoma metastasizes by different mechanisms, which include direct invasion of the surrounding tissue, spreading via the lymphatic vascular system as well as by haematological spreading. Currently, several factors are implicated as regulators of this different spreading. VEGF-C and VEGF-D are tumour-secreted cytokines, which bind to VEGF receptors on lymphatic endothelial cells leading to induction of new lymphatic capillaries Citation[3]. However, resent studies indicate that peri-tumoural lymphatic vessels induced by VEGF-C frequently exhibit abnormal, retrograde draining pattern, suggesting that VEGF-C induced lymphatics might play an important role in spreading of the tumour cells Citation[3].
Several markers of angiogenesis, such as endothelial protein receptor TIE-1, VEGF-C and vascular endothelial cadherin are over-expressed in melanomas Citation[20]. The expression of these genes is related to the phenomenon called vascular mimicry, in which highly aggressive tumour cells are able to undergo a genetic reversion to a pluripotent, more embryonic-like phenotype, suggesting that melanoma cells might be able to form new vessels to tumours by themselves Citation[20]. In renal cell cancer Zhang et al. Citation[21] have suggested that degree of angiogenesis is associated with progression and mediated by increased VEGF and MMP levels. Thus, neovascularization mechanisms in tumour cells may explain the aggressiveness of metastatic melanoma and provide a basis for specific therapy targeting angiogenic molecules and signal transduction pathways.
Serum concentrations of VEGF-C are 1 000–1 300 pg/ml in healthy individuals and can be elevated due to overweight Citation[22]. Our study is the first study on serum levels of VEGF-C in patients with metastatic melanoma. Metastatic site has an important impact on patient survival according to the AJCC melanoma classification system Citation[23]. One of the aims of the present study was to assess, whether VEGF-C levels might be of any value in correlating with the metastatic sites i.e., skin, subcutaneous tissues, superficial and deep lymph nodes, lung and liver.
In the present study, serum levels of VEGF-C were found to correlate with particular metastatic sites. Lower VEGF-C levels were significantly associated with the presence of skin or subcutaneous metastases. Furthermore, higher mean serum VEGF-C levels significantly predicted an increased risk for metastasis in the deep lymph nodes. It has been shown previously that melanoma patients with skin, subcutaneous or lymph node metastases have a one-year survival rate of 59%, and those with lung metastases 57% Citation[23]. These figures are markedly better, when compared to the patients, who have other visceral metastases, with one-year survival rate of only 41% Citation[23]. In our series, however, we could not establish any direct relationship between the serum VEGF-C levels and overall survival, even in univariate survival analysis.
Melanoma patients are not followed by regular CT-scans and therefore, it is possible that skin metastases are detected earlier than deep metastases. Due to this earlier diagnosis, it is possible that patients with skin and subcutaneous metastases only have a small tumour burden, while those with deep lymph node metastases have a larger tumour volume. However, in our study no direct association between tumor burden and serum VEGF-C levels could be seen. Half of the patients with only one metastatic site had their serum VEGF-C levels above median and the other half of the patients below median.
The importance of different metastatic sites in prognosis is known, but the molecular mechanisms underlying this phenomenon are obscure. Goydos and Gorski Citation[17] have studied melanoma tumours with quantitative real-time PCR and shown that in tumour progression, VEGF-C levels rise. However, they found that VEGF-C levels were equal in distant metastases and negative lymph nodes suggesting that VEGF-C is a special indicator of subcutaneous and lymph node metastasis Citation[17]. These findings are in accordance with the present results.
We found that in patients with visceral metastases, the serum levels of VEGF-C were higher, but non-predictive for the specific metastatic site. VEGF-C is also an independent risk factor for peritoneal metastases and correlates with the presence of ascites Citation[5], implicating that it has an important role in tumour dissemination beyond the local lymph nodes. The present results suggest that in melanoma, direct invasion or spreading to subcutaneous tissue is regulated by VEGF-C, whereas in visceral metastases which are mainly blood-borne, preferentially other factors determine the subsequent outcome.
Treatment results of metastatic melanoma are poor. Recent studies suggest that dacarbazine (DTIC), the “gold standard” treatment of metastatic melanoma, may lead to over-production of IL-8 and VEGF in melanoma cells Citation[24]. Over-expression of these cytokines leads to activation of mitogenic signals, with enhanced tumour growth and metastasis in vivo Citation[24]. Interestingly, over-expression of these cytokines is also related to resistance to DTIC treatment suggesting a possible, VEGF-mediated mechanism of escape from this standard chemotherapy Citation[24]. In the present study, all patients who had received adjuvant IFN-α therapy had lower serum VEGF-C levels, and they were randomized by chance to the BOLD and IFN-α treatment arm. We could not establish any direct relationship between VEGF-C levels and treatment outcome. The previously instituted adjuvant IFN-α therapy might have exerted an anti-angiogenic effect on the tumours in these patients, leading to down-regulation of VEGF-C, which may have also affected the treatment response. The mechanisms of action of IFN-α therapy in melanoma are not fully understood. A recent report by Gogas et al. Citation[25] shows that patients who develop with autoimmune reactions during treatment with IFN-α2b have better survival.
To conclude, several studies have implicated the importance of VEGF-C (analysed in tumours) in progression and metastatic dissemination of malignant melanoma. For the first time, our results suggest that serum VEGF-C levels might be associated with the development of skin or subcutaneous metastasis in patients with advanced melanoma. Measurement of VEGF-C might also have clinical value when evaluating the potency of new anti-angiogenic therapies in the treatment of patients with metastatic melanoma.
Acknowledgements
This study has been supported by the research grants from the Finnish Cancer Foundation, Finnish Cultural Foundation, and Turku University Hospital.
References
- Underiner TL, Ruggeri B, Gingrich DE. Development of vascular endothelial growth factor receptor (VEGFR) kinase inhibitors as anti-angiogenic agents in cancer therapy. Curr Med Chem 2004; 11: 731–45
- Ishii H, Yazawa T, Sato H, Suzuki T, Ikeda M, Hayashi Y, et al. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs). Lung Cancer 2004; 45: 325–37
- Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumour lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res 2004; 64: 4400–4
- Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer 2003; 88: 237–44
- Nishida N, Yano H, Komai K, Nishida T, Kamura T, Kojiro M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 2004; 101: 1364–74
- Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Tamaya T. Clinical implication of expression of vascular endothelial growth factor-C in metastatic lymph nodes of uterine cervical cancers. Br J Cancer 2004; 91: 466–9
- Branca M, Giorgi C, Santini D, Di Bonito L, Ciotti M, Benedetto A, et al. Aberrant expression of vascular endothelial growth factor-C (VEGF-C) is related to grade of cervical intraepithelial neoplasia (CIN) and high-risk Human papillomavirus (HPV), but does not predict virus clearance after treatment of CIN or prognosis of cervical cancer. J Clin Pathol 2006; 59: 40–7
- Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer 2004; 46: 11–9
- Tamura M, Oda M, Tsunezuka Y, Matsumoto I, Kawakami K, Ohta Y, et al. Chest CT and serum vascular endothelial growth factor-C level to diagnose lymph node metastasis in patients with primary non-small cell lung cancer. Chest 2004; 126: 342–6
- Ishikawa M, Kitayama J, Kazama S, Nagawa H. The expression pattern of vascular endothelial growth factor C and D in human esophageal normal mucosa, dysplasia and neoplasia. Hepatogastroenterology 2004; 59: 1319–22
- Price N. Vascular endothelial growth factors C and D in colorectal cancers. Clin Colorectal Cancer 2004; 1: 23–5
- Choi WW, Lewis MM, Lawson D, Yin–Goen Q, Birdsong GG, Cotsonis GA, et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: Correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol 2005; 18: 143–52
- Tamura M, Ohta Y. Serum vascular endothelial growth factor-C level in patients with primary nonsmall cell lung carcinoma: A possible diagnostic tool for lymph node metastasis. Cancer 2003; 98: 1217–22
- Tamura M, Oda M, Matsumoto I, Tsunezuka Y, Kawakami K, Ohta Y, et al. The combination assay with circulating vascular endothelial growth factor (VEGF)-C, matrix metalloproteinase-9, and VEGF for diagnosing lymph node metastasis in patients with non-small cell lung cancer. Ann Surg Oncol 2004; 11: 928–33
- Mathur SP, Mathur RS, Gray EA, Lane D, Underwood PG, Kohler M, et al. Serum vascular endothelial growth factor C (VEGF-C) as a specific biomarker for advanced cervival cancer: relationship to insulin-like growth factor II (IGF-II), IGF binding protein 3 (IGF-BP3) and VEGF-B. Gynecol Oncol 2005; 98: 467–83
- Mitsuhashi A, Suzuka K, Yamazawa K, Matsui H, Seki K, Sekiya S. Serum vascular growth factor (VEGF) and VEGF-C levels as tumour markers in patients with cervical carcinoma. Cancer 2005; 103: 724–30
- Goydos JS, Gorski DH. Vascular endothelial growth factor C mRNA expression correlates with stage of progression in patients with melanoma. Clin Cancer Res 2003; 9: 5962–7
- Schietroma C, Cianfarani F, Lacal PM, Odorisio T, Orecchia A, Kanitakis J, et al. Vascular endothelial growth factor-C expression correlates with lymph node localization of human melanoma metastases. Cancer 2003; 98: 789–97
- Vuoristo MS, Hahka-Kemppinen M, Parvinen LM, Pyrhönen S, Seppä H, Korpela M, et al. Randomized trial of dacarbazine versus bleomycin, vincristine, lomustine and dacarbazine (BOLD) chemotherapy combined with natural or recombinant interferon-alpha in patients with advanced melanoma. Melanoma Res 2005; 15: 291–6
- Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vascular mimicry and tumour-cell plasticity. Lessons from melanoma. Nature Rev Cancer 2003; 3: 411–21
- Zhang X, Yamashita M, Uetsuki H, Kakehi Y. Angiogenesis in renal cell carcinoma: Evaluation of microvessel density, vascular endothelial growth factor and matrix metalloproteinases. Int J Urol 2002; 9: 509–14
- Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obesity 2005; 29: 1308–14
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19: 3635–48
- Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: A possible escape mechanism from chemotherapy. Mol Cancer Ther 2003; 8: 753–63
- Gogas H, Ionnovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006; 354: 709–18