Abstract
The aim of this study was to determine the clinical impact of whole body positron emission tomography (FDG PET) to detect clinically silent metastases in the follow-up of patients with high risk melanoma. FDG PET was performed to 30 asymptomatic melanoma patients (AJCC stage IIB–IIIC) 7–24 months after the primary surgery and sentinel node biopsy. FDG PET was able to detect six of seven recurrences, constituting 20% of all study patients. One patient presented with a negative FDG PET finding at the very first scanning, but was positive later in a repeated scan after manifestation of palpable mass in the axilla. The positive PET finding had an impact on treatment decisions in every case: three patients underwent surgical resection and four patients received chemotherapy or interferon. The mean follow-up time was 27 months (range, 12–48 months) and during that time the other 23 patients with true negative FDG PET were disease-free. One of the seven recurrences was in remission after surgical metastasectomy. In conclusion, whole body FDG PET is a valuable follow-up tool in high risk melanoma to diagnose recurrences and to select the patients, who are suitable for surgical metastasectomy.
In Finland the incidence of melanoma has been steadily increasing. According to the Finnish Cancer Registry, the incidence rate of melanoma was 8.9 among male and 7.7 among female in 1999–2003 (age-adjusted rate per 100 000 population). During the same period the mortality rate of melanoma was 2.1 in male and 1.1 in female, respectively Citation[1].
A proportion of cutaneous melanoma patients carry a risk for developing recurrence and distant metastases. The probability for metastatic spreading can be estimated by the prognostic factors based on the histology of the primary lesion and nodal status. In this study a high risk melanoma is defined as stage IIB (tumour thickness 2.01–4.0 mm with ulceration or >4.0 mm without ulceration), stage IIC (tumour thickness >4.0 mm with ulceration) or stage III (any tumour thickness with nodal metastases present) melanoma, according to the staging system of American Joint Committee on Cancer (AJCC). In the AJCC database which consisted of 17 600 patients, these subgroups had 10 year survival rates varying between 51–54%, 32% and 15–63%, respectively Citation[2].
Whole body [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET) is a non-invasive, high-resolution molecular imaging technique that can detect metastases of malignant melanoma, based on abnormal cellular glucose uptake. Overall, whole-body FDG PET is highly sensitive and specific for melanoma staging with the exception of the brain Citation[3], Citation[4]. However, at the time of the initial disease staging FDG PET seems to have suboptimal sensitivity for detection of both regional lymph node and distant metastases Citation[5], Citation[6]. This is due to its limited sensitivity to detect low tumour volume at the stage of microscopic disease Citation[7]. Therefore, current standard for the initial nodal staging of melanoma is sentinel node biopsy (SNB) which is a precise method to detect microscopic nodal involvement and conveys information on potential risk for locoregional or distant recurrence Citation[8], Citation[9].
Whenever occult disease has initially been left undetected the metastases at some stage will grow and FDG PET then has a higher potential for staging purposes. Many studies have shown that the recurrence of melanoma most commonly occurs during the first 2–3 years after initial surgery Citation[10], Citation[11]. The optimal timing of FDG PET should take into account this window when clinical disease progression shows the highest likelihood. In the Turku University Hospital in Finland, some 60 patients with new melanoma are treated annually; the catchment population is 450 000 residents within the hospital district. The aim of this prospective study was to determine the clinical impact of FDG PET to detect clinically silent metastases in the follow-up of patients with high risk melanoma.
Material and methods
Patients
From March 2004 to November 2005, 30 AJCC stage IIB–IIIC adult melanoma patients were enrolled in this prospective study in the Turku University Hospital, Turku, Finland. The patients were selected consecutively from a prospective melanoma database, founded at our institution in October 2001 for registering all sentinel node biopsies. Patient and primary tumour characteristics are given in . All patients were free of any clinical signs of metastasis at the time of study inclusion. None of the patients refused to participate in the study. The study protocol was approved by the local institutional ethical review committee and informed consent was obtained from each patient.
Table I. Characteristics of study patients.
A whole body FDG PET scanning was performed 7–24 months after the primary surgery, independently from the regular follow-up schedule which consists of whole body computed tomography (CT) at the time of initial surgery and clinical examination every 3–6 months during the first five years. Routine chest-X-ray and blood tests including ;iver chemistry were performed annually. A secondary CT and physical examination were performed concurrently with FDG PET and the median interval between FDG PET and CT was 35 days (range, 1–145 days). CT-scan covered the same body level as FDG PET in each case. All patients had undergone lymphatic mapping with attempt for SNB (n = 30) and subsequently 15 of these had underwent further radical lymph node dissection based on nodal metastasis. One of the 15 patients who had nodal dissection and confirmed stage III disease had macroscopic nodal involvement while in the remaining 14 patients micrometastasis was found only in the sentinel node(s). Only one of 30 patients had undergone an unsuccessful lymphatic mapping with no radioactive lymph nodes and her disease was initially judged as stage IIB according to the status of the primary lesion. No patient had had any adjuvant therapy after initial surgery.
FDG PET imaging
Patients fasted at least 6 hours before entering Turku PET Centre, where all studies were performed. The imaging device was GE Advance (General Electric Medical Systems, Milwaukee, WI, USA) or CTI ECAT HR+ (Siemens Medical Systems, Knoxville, TN, USA) PET scanner which both operated in 2-D mode. The GE Advance and HR+ scanners consist of 18 and 32 rings of bismuth germanate detectors (BGO) yielding 35 and 63 transverse slices spaced by 4.25 mm and 2.46 mm, respectively. The imaging field of view is 55 cm in diameter in both scanners and 15.2 cm (GE Advance) and 15.5 cm (HR + ) in axial length. A bolus of approximately 370 MBq of FDG was injected through a venous catheter which was flushed with saline after tracer injection. Blood glucose was evaluated routinely with HaemoGlucotest®.
After waiting period when physical activity was minimized the patients were placed supine on scanner couch with arms downwards. Static PET imaging covering the entire body in case of lower extremity primary or the upper torso from eyebrows to mid thighs in case of abdominal, thoracic, head and neck, and upper extremity primaries started 50 min after FDG injection (5 min emission scan/position). To correct for photon attenuation a 2 min post-emission transmission scan was performed with robotically operated 68Ge rods.
PET image analysis
Image analysis was performed by a certified nuclear medicine physician with experience in FDG PET. All images were analyzed visually and any abnormal focal FDG activity was considered as positive for tumour if physiologic uptake e.g. in urinary system, heart, brain or gastrointestinal activity could be ruled out. In challenging cases consensus reading was performed by two physicians and only lesions deemed as suspicious for tumour were classified as positive. Anatomical reference CT images were used to define the exact site of pathologic accumulation before final scan interpretation.
Follow-up
The follow-up time for each patient was defined as the period from the primary surgery to the death or to the closing date of this study, March 31, 2006. FDG PET was not repeated routinely, if the first scan was negative and if the patient remained asymptomatic.
Results
The mean follow-up time is currently 27 months (range, 12–48 months) from the time point of initial surgery. The mean time from primary surgery to the FDG PET was 11 months (range, 7–24 months) in all study patients and 9 months (range, 7–14 months) in patients revealing a true positive FDG PET finding.
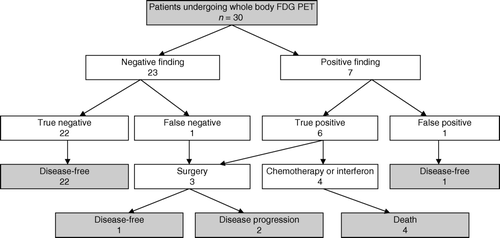
There were seven recurrences in the study population (23%). Six of them were upstaged by FDG PET (20% of all study patients) and one patient presented with a negative finding at the first scanning, but positive finding in a repeated scan after manifestation of palpable mass in the axilla. This case is regarded as a false negative while six others were true positive on baseline FDG PET. The data on the seven recurrences are summarized in . The anatomic localisation of metastases was subsequently verified by CT in every case. Histological confirmation of melanoma recurrence was obtained in three cases, and in the remaining cases metastatic disease was judged by subsequent clinical disease progression with appearance of concurrent findings on CT-scan.
Table II. Patients with recurrent disease.
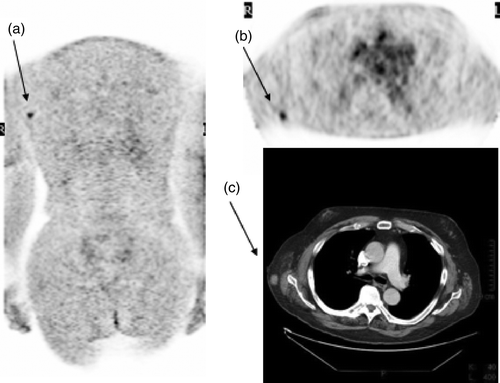
The recurrence had influence on the treatment strategy in all patients. Three patients underwent surgery with a curative intent. An obese patient with no palpable disease demonstrated subcutaneus intransit metastasis of trunk on FDG PET () and underwent wide soft tissue excision with local flap reconstruction. The second patient underwent thoracotomy and lobectomy because of a bifocal lung metastasis. The third patient underwent axillary clearance of nodal metastases. Four patients with inoperable recurrent disease received chemotherapy and/or interferon. There were four melanoma deaths during the follow-up period. In those patients the disease was upstaged by FDG PET when they were asymptomatic but all of them experienced a rapid progression of disease which showed poor response to chemotherapy.
Figure 1. 60-year-old male operated previously due to stage IIB melanoma in the back. FDG PET coronal slice (a, arrow) and transaxial slice (b, arrow) revealed an FDG-avid lesion near the right axilla (scanned arms down) corresponding a small (10 mm) tumour in diagnostic CT, arms up (c, arrow). Histological study confirmed a melanoma metastase.
One patient had bifocal hypermetabolic activity in the mediastinum, but there was no progression of this equivocal finding in the repeated PET scans after 3 and 6 months. Also CT was normal and the PET finding was justified to have a benign underlying cause.
This resulted in a sensitivity and specificity for melanoma recurrence of 86% and 96%, respectively. The positive predictive value was 86% and the negative predictive value was 96%. The entire study profile is summarized in .
Discussion
Whole body FDG PET is a potential tool to detect clinically silent metastases in high risk melanoma during the postoperative follow-up. A Danish study has recently demonstrated that occult metastases were found with FDG PET in 12% among stage III sentinel node positive melanoma patients Citation[12].
In general, no universally accepted consensus has been adopted thus far for the routine use of FDG PET in the follow-up of melanoma Citation[13]. The major benefit of using FDG PET for melanoma is the detection of recurrent disease at a potentially curable stage Citation[14]. The earlier the metastasis is detected, the more effective the treatment is likely to be accomplished Citation[15]. Surgery is the only modality of therapy that significantly influences the prognosis Citation[16]. Single distant metastases are suitable for surgical resection and results have been reported to give some therapeutic benefit in terms of prolonged survival Citation[17], Citation[18].
However, as a screening method in asymptomatic melanoma patients the routine use of FDG PET is controversial. In many national guidelines no routine imaging modalities are recommended in the follow-up of stage I–II melanoma Citation[19], Citation[20]. On the other hand, stage I–II constitute prognostically a heterogeneous group and a fraction of these patients will have an aggressive disease and a considerable risk of developing advanced stage disease. If the primary lesion is thick or ulcerated, the prognosis can be even worse than in some subgroups of stage III Citation[2]. Therefore, stage IIB and stage IIC melanomas should also be included in the high risk category. In this study three recurrences were detected in stage III group (20% of all stage III patients) and four in stage II group (27% of all stage II patients), respectively.
If FDG PET is considered as a screening method, attention has to be paid to acceptable detection rate and impact on treatment decision Citation[21–23]. In this study FDG PET revealed clinically silent metastases in 20% of cases and this upstaging influenced management in every case. Three patients underwent surgical metastasectomy and four patients received chemotherapy and/or interferon. One patient is currently disease-free after surgical metastasectomy, but the final survival benefit of the PET imaging cannot be estimated due to the limited follow-up time.
All melanoma recurrences were found with FDG PET between 7–14 months after the initial disease presentation suggesting the optimal timing for FDG PET may be award 6 months after the initial disease presentation. Obviously, the optimal time point is not certain, since each patient carries an unpredictable and individual course of the disease progression. In this study no metastasis was found later than 14 months after initial surgery. However, ten patients with negative FDG PET have had a total follow-up period less than 24 months which is rather short. We want to stress the importance of intensive follow-up even if the FDG PET is negative, due to the risk of late recurrence. One study patient had a negative FDG PET seven months after surgery, but developed clinically evident nodal recurrence seven months later and a repeated FDG PET had changed to positive at that stage. In addition, it was the only recurrence in the regional nodal basin preceded by an unsuccessful SNB at the time of primary surgery. We have earlier reported a 96% sentinel node detection rate in melanoma Citation[24]. A failed SNB could therefore constitute a special indication for FDG PET provided that the primary lesion is thick or of intermediate thickness with ulceration. In some cases a massively invaded sentinel node may be detected by PET but missed by lymphoscintigraphy Citation[25].
The false positive findings bring an additional problem. 18F-FDG is a non-specific metabolic agent that reflects increased cellular glucose metabolism regardless of the underlying cause. In this study one borderline PET finding lead on to repeated PET scans until 6 months later the finding was classified as true negative. Obviously, in such case there is no consensus regarding the extent and duration of follow-up necessary to validate a true negative finding. If such borderline findings are associated with limited chance to histologic biopsy, CT or MRI may be helpful as complementary imaging modalities. Due to the apparent risk for false positive cases we argue that neither chemotherapy nor surgery should be based on equivocal or unverified PET finding.
In Finland, the limited availability has thus far barred wider acceptability of FDG PET in clinical practice. Meanwhile, the incidence of cutaneus melanoma is rising and over 700 new cases are diagnosed annually. This fact and increasing overall cancer incidence in the aging population poses challenges in future use of clinical FDG PET in Finland. Currently diagnostic imaging with hybrid PET/CT scanners are provided in two tertiary hospitals and a mobile PET unit offers biweekly services to the rest of the country. Since a third scanner will soon be installed in a third hospital it is evident that within the few next years limited availability will not pose a problem for PET imaging of melanoma and other cancer patients. Our study has demonstrated in accordance with several others Citation[26–28] the feasibility of FDG PET in management of high-risk melanoma and our findings warrant evaluation of cost-effectiveness studies where FDG PET is put in the diagnostic algorithm in the follow-up phase of malignant melanoma.
In conclusion, whole body FDG PET is a valuable follow-up tool in high-risk melanoma to diagnose recurrences. During a postoperative period between 7–14 months FDG PET revealed most of all recurrences at an early asymptomatic stage. On the other hand, negative FDG PET finding seems to be a good prognostic sign. Furthermore, FDG PET has an important role in all melanoma patients where surgical treatment is considered to exclude more advanced-stage disease.
References
- URL:, http://www.cancerregistry.fi/eng/statistics/.
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19: 3635–48
- Larcos G, Maisey MN. FDG-PET screening for cerebral metastases in patients with suspected malignancy. Nucl Med Commun 1996; 17: 197–8
- Ludwig V, Komori T, Kolb D, Martin WH, Sandler MP, Delbeke D. Cerebral lesions incidentally detected on 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography images of patients evaluated for body malignancies. Mol Imaging Biol 2002; 4: 359–62
- Wagner JD, Schauwecker D, Davidson D, Logan T, Coleman JJ 3rd, Hutchins G, et al. Inefficacy of F-18 fluorodeoxy-D-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer 2005; 104: 570–9
- Havenga K, Cobben DC, Oyen WJ, Nienhuijs S, Hoekstra HJ, Ruers TJ, et al. Fluorodeoxyglucose-positron emission tomography and sentinel lymph node biopsy in staging primary cutaneous melanoma. Eur J Surg Oncol 2003; 29: 662–4
- Wagner JD, Schauwecker DS, Davidson D, Wenck S, Jung SH, Hutchins G. FDG-PET sensitivity for melanoma lymph node metastases is dependent on tumor volume. J Surg Oncol 2001; 77: 237–42
- Belhocine T, Pierard G, De Labrassinne M, Lahaye T, Rigo P. Staging of regional nodes in AJCC stage I and II melanoma: 18FDG PET imaging versus sentinel node detection. Oncologist 2002; 7: 271–8
- Acland KM, Healy C, Calonje E, O'Doherty M, Nunan T, Page C, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of micrometastases of primary cutaneous malignant melanoma. J Clin Oncol 2001; 19: 2674–8
- Dicker TJ, Kavanagh GM, Herd RM, Ahmad T, McLaren KM, Chetty U, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. Br J Dermatol 1999; 140: 249–54
- Fusi S, Ariyan S, Sternlicht A. Data on first recurrence after treatment for malignant melanoma in a large patient population. Plast Reconstr Surg 1993; 91: 94–8
- Horn J, Lock-Andersen J, Sjøstrand H, Loft A. Routine use of FDG-PET scans in melanoma patients with positive sentinel node biopsy. Eur J Nucl Med Mol Imaging 2006; 33: 887–92
- Poo-Hwu WJ, Ariyan S, Lamb L, Papac R, Zelterman D, Hu GL, et al. Follow-up recommendations for patients with American Joint Committee on Cancer Stages I-III malignant melanoma. Cancer 1999; 86: 2252–8
- Damian DL, Fulham MJ, Thompson E, Thompson JF. Positron emission tomography in the detection and management of metastatic melanoma. Melanoma Res 1996; 6: 325–9
- Garbe C, Paul A, Kohler-Spath H, Ellwanger U, Stroebel W, Schwarz M, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: Recommendations for an effective follow-up strategy. J Clin Oncol 2003; 21: 520–9
- Brand CU, Ellwanger U, Stroebel W, Meier F, Schlagenhauff B, Rassner G, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma. An analysis of related prognostic factors. Cancer 1997; 79: 2345–53
- Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol 1999; 71: 209–13
- Essner, R, Lee, JH, Wanek, LA, Itakura, H, Morton, DL. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 2004;139:961–6; Discussion 966–7.
- Bishop JA, Corrie PG, Evans J, Gore ME, Hall PN, Kirkham N, et al. UK guidelines for the management of cutaneous melanoma. Br J Plast Surg 2002; 55: 46–54
- Australian Cancer Network:. Guidelines for the management of cutaneous melanoma. Stone Press, Sydney 1997
- Gulec SA, Faries MB, Lee CC, Kirgan D, Glass C, Morton DL, et al. The role of fluorine-18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: Impact on surgical decision making. Clin Nucl Med 2003; 28: 961–5
- Tyler DS, Onaitis M, Kherani A, Hata A, Nicholson E, Keogan M, et al. Positron emission tomography scanning in malignant melanoma. Cancer 2000; 89: 1019–25
- Bastiaannet E, Oyen WJ, Meijer S, Hoekstra OS, Wobbes T, Jager PL, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography on surgical management of melanoma patients. Br J Surg 2005; 93: 243–9
- Koskivuo I, Suominen E, Niinikoski J, Talve L. Sentinel node metastasectomy in thin <or = 1-mm melanoma. Langenbecks Arch Surg 2005; 390: 403–7
- Blocklet D, Donckier V, Vereecken P, Van Geertruyden J, Laporte M, Goldman S. Nondetection of sentinel lymph node with lymphoscintigraphy as a result of massive malignant invasion shown by positron emission tomography. Clin Nucl Med 2001; 26: 1013–5
- Rinne D, Baum RP, Hor G, Kaufmann R. Primary staging and follow-up of high risk melanoma patients with whole-body 18F-fluorodeoxyglucose positron emission tomography: results of a prospective study of 100 patients. Cancer 1998; 82: 1664–71
- Holder, WD, Jr, White, RL, Jr, Zuger, JH, Easton, EJ, Jr, Greene, FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg 1998;227:764–9; Discussion 769–71.
- Brady MS, Akhurst T, Spanknebel K, Hilton S, Gonen M, Patel A, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Oncol 2006; 13: 525–32