Abstract
The APC gene plays an important role in colorectal carcinogenesis. The impact of APC mutations on the clinical features in sporadic CRC remains to be uncovered. The APC gene was screened for mutations with systematic analysis techniques including DHPLC, PTT, MLPA and DNA sequencing in 43 Chinese sporadic CRC patients. Twenty nine somatic mutations (in 17 different types) in APC gene were found in 18 of 43 sporadic CRC patients. Of those, nine were novel mutations. Higher frequency of somatic APC mutations was found in younger CRC patients than that in elder ones. The biallelic somatic mutations of APC gene were identified in four CRC patients whose tumors had more invasive clinical features. The nonsense mutation Arg1114X in APC gene was found in five of 43 CRC tumor tissues. A higher cancer metastasis rate was uncovered in CRC patients with this mutation. The somatic mutations of APC gene may influence the clinical features of sporadic CRC. Arg1114X in APC gene, as a hot spot mutation in Chinese CRC, may predispose to the cancer metastasis of sporadic CRC.
The APC gene acts as a gate-keeper for the development of colorectal cancers (CRC). Most of CRC are thought to follow a genetic pathway involving APC. Mutations in the APC gene are considered as one of the earliest events both in the initiation and progression of sporadic CRC and FAP-associated disease Citation[1], Citation[2].
The APC has been regarded as a classical Knudson-type tumor suppressor gene. It is generally accepted that tumors start to grow in the colorectum of the FAP patients when an appropriate cell acquires a somatic mutation at the APC locus to accompany a preexisting germline mutation. In part of the sporadic tumors of the colorectum, two inactivating APC mutations often occur, one leading to a truncated protein and the other being a similar or changed mutation resulting in allelic loss (so called biallelic APC mutation) Citation[3], Citation[4]. However, whether the biallelic APC mutations have impact on the progression of the tumors in sporadic CRC remains to be proved.
It has been reported that the clinical variability in FAP might be related to the positions of germline mutations in APC gene Citation[5–9]. However, the relationship between somatic mutations of APC gene in tumor tissues and clinical features of sporadic CRC has seldom been investigated. It is necessary to uncover the mutation spectrum of the APC gene in sporadic CRC if a clue about the “genotype-phenotype” correlation of APC gene in sporadic CRC were expected.
The mutation frequency and spectrum of APC gene in sporadic CRC varies among studies Citation[10]. Two reasons might be considered, the first is that the APC gene is composed of 8 529 base pairs from 15 exons in genomic DNA. It is comprehensively difficult to perform a completed sequence screening of this gene. The second, mutation cluster region (MCR) reported in APC gene has understandably conducted some studies to concentrate mutation checking in this region. Here we scan the APC gene in sporadic CRC by several techniques to show the frequency and spectrum of APC mutations and compare the advantages of different techniques in mutation screening in the APC gene. And at the same time, correlation of clinical features and APC mutations in sporadic CRC would also be analyzed.
Materials and methods
Subjects
A total of 43 index patients with sporadic CRC had undergone tumor resection before receiving chemotherapy or radiotherapy. Ages of these patients ranged from 31 to 77 years old (mean age 57 years). Thirteen of them were diagnosed at a younger age (<50 years old), 23 around mean age (from 50 to 69 years old) and seven were older (≥70 years old). All of CRC patients in this study had been diagnosed by histopathology.
With regard to tumor localization, two anatomical sites were considered: Sixteen of them were colon cancers and 27 rectal cancers. Tumor grading was defined according to WHO directives, 35 of them presented middle differentiated adenocacinoma (II) and eight low differentiated (III). All cases were staged using a modified Dukes’ system: A, cancer limited to bowel wall (4 cases); B, with extramural spread (24 cases); C, with lymph node metastasis (14 cases); D, with distant metastasis (1 case). We also evaluated the pattern of cancer growth for the samples studied: expanding (17 cases) or infiltrating (26 cases).
Informed consent was obtained from all patients included in this study.
Sample collection
Genomic DNA was extracted from both normal colorectal tissues and tumor tissues of sporadic CRC patients for screening both germline and somatic mutations in APC gene.
Mutation analysis
Three techniques were used to analyze different kinds of mutations in the APC gene.
Denaturing high-performance liquid chromatography (DHPLC)
DHPLC is a sensitive technique with high throughout-put to detect small mutations (small deletions or insertions leading to frameshifts, splice site alterations, nonsense and missense mutations) in DNA fragments with length of 100–500 bp. The exons 1 to 14 of APC gene, each of them in range of 100–400 bp, were analyzed by DHPLC (WAVE system, Transgenomic). Oligonucleotide primers used in PCR for DHPLC had been reported previously Citation[11]. Assay conditions (acetonitrile gradient and temperature profile) were optimized for DHPLC of DNA fragment with a bank of samples previously characterized by DNA sequence. The PCR products which showed variant peaks in DHPLC were analyzed by direct sequencing (ABI 3100, Applied Biosystems).
Protein truncation test (PTT)
PTT can sensitively detect a truncating mutation in gene fragments of up to 2 kb in one performance and is commonly used to check mRNA for mutations. Exon 15 of APC gene is composed of 6 571 bp. PTT that allows the detection of truncated protein was performed with genomic DNA to screen mutation in this exon of APC gene in higher efficiency. Exon 15 was amplified in four overlapping fragments from genomic DNA templates by primers that had been reported Citation[11], Citation[12]. Resultant PCR products were used in a coupled transcription-translation reaction (Promega) incorporating 35S-labeled methionine. Labeled protein products were analyzed by 8% SDS/PAGE. The samples showing truncated protein products were analyzed by direct sequencing for characterization of the mutations (ABI 3100, Applied Biosystems).
Multiplex ligation-dependent probe amplification (MLPA)
MLPA was used to detect the large fragment deletion or duplication in APC gene in the checked samples on the reason that the large fragment variations in APC gene were suggested to represent a frequent cause of FAP Citation[7], Citation[13]. The mixture of probes in MLPA contains 20 probes for test APC gene (two of them for the promotor region, one for the 5'untranslated sequence, 14 for exon 1–14, and three for exon 15 of APC gene), and 11 control probes specific for DNA sequences outside the APC gene (MRC-Holland). MLPA was performed according to the protocol supplied with the kit.
Statistic analysis
The statistic evaluations were performed with Nonparametric test (Wilcoxon rank test) and χ2 test (Fisher's exact test). A significant difference was considered if p-value (two-tail) was less than 0.05.
Results
The combined use of DHPLC, PTT, MLPA and DNA sequencing for mutation analysis in APC gene allowed the detection of 29 somatic mutations in 18 samples of 43 sporadic CRC patients. No germline mutation of APC gene was discovered in sporadic CRC patients.
Somatic mutations of APC gene in sporadic CRC
In this study, we identified 29 somatic mutations (in 17 different types) in the tumors of 18/43 (42%) patients with sporadic CRC (). Among the 18 patients with APC gene somatic mutations, 11 of them have only one mutation and seven have 2–4 mutations in APC gene. The mutations detected in this study were distributed in exons 3, 9, and 15. Of 17 mutation types detected in this study, eight (about 48%) were deletions or insertions (one of them was 91 bp deletion uncovered by PTT in exon 15 of APC gene), and nine (52%) were single-base pair substitutions resulting in a nonsense mutation. Interestingly, near 80% of the single-base pair substitutions showed a transition of C:G to T:A. All of the mutations would introduce premature termination signals from which a truncated protein of APC gene was produced. Nine of the mutations detected in this study had not been presented in the APC mutation database so far (http://www.hgmd.cf.ac.uk).
Table I. Somatic APC mutations detected in tumors of sporadic CRCs.
No large genomic deletion was detected in APC gene in tumor tissues checked by MLPA. We also checked the DNA samples from normal colon tissues of these patients in whom somatic mutation had been unveiled in their tumor tissues. No germline mutation was found in them.
Relationship between somatic mutations in APC gene and the clinical features of sporadic CRC
Different frequencies of somatic APC gene mutations were uncovered among the groups on the age of CRC diagnosis (). Statistical analysis showed that a significantly higher frequency of somatic mutations in APC gene existed in the tumors of younger CRC patients (Wilcoxon rank test: p = 0.048). A negative correlation was unveiled between the diagnosis age of patients disease onset and the mutation frequency found in APC gene (Pearson correlation coefficients: r = − 0.382, p = 0.012).
Table II. Correlation between APC mutations and the age of diagnosis of the CRC.
The correlation between the somatic mutations of APC gene and the clinical/pathological features of sporadic CRC patients were analyzed as well. Of 18 CRC patients with somatic mutation of APC gene, seven were detected to have two or more mutations. With the results of PTT, four of them (CRC4, 18, 32, 33) were identified as harboring biallelic mutations in the exon 15 of APC gene. However, the biallelic mutations could not be confirmed in the other three patients with two or more somatic mutations in APC gene. All of the four CRC patients with biallelic mutations of APC gene were at early diagnosis ages (<50 years) and have a similar infiltrating pattern of cancer growth (). A higher frequency of somatic mutation in APC gene was found in the CRC patients with tumor of Dukes’ stage C/D when comparing with the patients with tumor of Dukes’ stage A/B (Fisher's exact test: p = 0.03). On the other hand, a less frequency of somatic mutation in APC gene was found in the tumor tissues with expanding growth pattern than that with infiltrating pattern (Fisher's exact test: p = 0.001). But, no significant difference of the frequency of somatic APC mutations was found between colon and rectal cancers (p = 0.207), and between different tumor grades (p = 1.000).
Table III. Correlation between APC mutations and the age/pathological features of tumors.
The nonsense mutation at codon 1114 (Arg1114X) in APC gene was frequently uncovered in this study. It was found in 5 of 43 (11.6%) tumor tissues. All of the patients with this mutation had their cancer metastasis (). Statistical analysis showed that a significant difference of cancer metastasis rate existed between the CRC patients with and without this mutation in APC gene (p = 0.036).
Table IV. Correlation between mutation at codon1114 and the metastasis status of the tumors.
Discussion
An effective analysis system is essential to complete mutation screening and molecular diagnosis due to the diversity of APC gene variations. In this study, combination of three methods (DHPLC, PTT and MLPA) for mutation analysis in APC gene is based on the characteristics of the three techniques and the structure of the APC gene. Variable frequencies of APC mutations have been reported in human CRC, ranging from 40% to 80% Citation[9], Citation[10], Citation[14–16]. The differences of mutation frequency in the literatures were probably explained by variations in the methodologies used to screen mutations in APC gene.
In our results, nearly 30% of the somatic mutations were deletions, 18% and 52% were insertions and single-base pair substitutions respectively. Most of deletions and insertions (nearly 70%) in the APC gene were observed at positions containing repeated sequences and nearly 74% of single-base pair substitutions were C:G to T:A transitions. It is similar to the results on the western CRC patients Citation[10], Citation[16–19]. As we know, the high frequency of deletion and insertion at the positions of repeated sequences suggested to be caused by DNA replication errors, and deamination of 5-methylcytosine in the CpG dinucleotide had been implicated as a cause for the transition of C:G to T:A. So a problem facing now is that whether the type of APC mutations in sporadic CRC is affected by some other genes, or only decided by the impact of environmental carcinogens. Further work need to be done to elucidate the mechanism of these mutations.
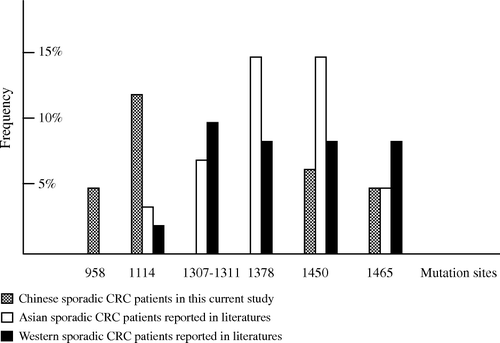
However, a difference in the pattern of distribution of APC mutations was observed between Chinese and western CRC patients (). Compared with the results from western sporadic CRC patients, the truncating mutations at codon 958 and 1114 detected in this study were at a distinct high frequency in Chinese sporadic CRC patients. On the other hand, mutations at codon 1309, 1307 and 1465 that were frequently found as germline and somatic mutations in FAP and CRC patients had not been detected in this work. Lower frequencies of these mutations might impute to the small sample set here. Additionally, whether mutations at codon 958 and 1114 are the hot spots in Chinese CRC should be confirmed by work on a larger sample set in the future.
It is generally accepted that inactivation of both alleles at the APC locus is required for development of most tumors in the colon and rectum. Inactivation of the APC gene by two (even more) mutations was also observed in sporadic CRC tumors in this study. Higher frequency of somatic APC mutations, even with more than one mutation, in the young CRC than that in the elder CRC patients suggested that a genomic instability might exist in the cells of young CRC patient. In this study, the patients with biallelic somatic mutations in tumor tissue had more invasive clinical features, including early age of disease onset, infiltrating pattern of cancer growth and Dukes’ stage C/D of tumors. These indicated that the functional state of APC gene in CRC would affect the tumor biological characteristics.
Although accumulated works has been done on the somatic APC mutation analysis in CRC patients, a few of them were on the relation to clinical features of CRC and the mutations detected Citation[19]. However similar work has seldom been done in Chinese CRC patients. The results in this study showed that somatic mutation at codon 1114 may be a hot spot in Chinese sporadic CRC and patients with this mutation showed a higher rate of cancer metastasis. As we know, the sequence between codon 1020 and 1169 of APC gene translated the first domain of APC protein for interaction with β-catenin which is important for activation of E-cadherin, a Ca(+ + )-dependent adhesion molecule that controls cell motility by affecting cell migration and morphogenesis Citation[20], Citation[21]. Our result suggests that mutations occurring in this motif may expedite the tumor metastasis in sporadic CRC. Nevertheless, further work should be performed on the functional significance of this mutation to confirm whether it can be consider as a molecular marker for CRC metastasis.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (No: 30470959) and the Health Department, Jiangsu Province, P. R. China (H0207, RC200207). Xiaorong Liu was a recipient of a China Scholarship Council (CSC) and Deutscher Akademischer Austauschdienst (DAAD).
References
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992; 359: 235–7
- Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol 2000; 18: 1967–79
- Cheadle JP, Krawczak M, Thomas MW, Hodges AK, Al-Tassan N, Fleming N, et al. Different combinations of biallelic APC mutation confer different growth advantages in colorectal tumours. Cancer Res 2002; 62: 363–6
- Lamlum H, Papadopoulou A, Ilyas M, Rowan A, Gillet C, Hanby A, et al. APC mutations are sufficient for the growth of early colorectal adenomas. Proc Natl Acad Sci 2000; 97: 2225–8
- Caspari R, Friedl W, Mandel M, Moslein G, Kadmon M, Knapp M, et al. Familial adenomatous polyposis: Mutation at codon 1309 and early onset of colon cancer. Lancet 1994; 343: 629–32
- Friedl W, Meuschel S, Caspari R, Lamberti C, Krieger S, Sengteller M, et al. Attenuated familial adenomatous polyposis due to a mutation in the 3'part of the APC gene. A clue for understanding the function of the APC protein. Hum Genet 1996; 97: 579–84
- Sieber OM, Lamlum H, Crabtree MD, Rowan AJ, Barclay E, Lipton L, et al. Whole-gene APC deletions cause classical familial adenomatous polyposis, but not attenuated polyposis or “multiple” colorectal adenomas. Proc Natl Acad Sci 2002; 99: 2954–8
- Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet 1998; 62: 1290–301
- Nagase H, Miyoshi Y, Horii A, Aoki T, Petersen GM, Vogelstein B, et al. Screening for germ-line mutations in familial adenomatous polyposis patients: 61 new patients and a summary of 150 unrelated patients. Hum Mutat 1992; 1: 467–73
- Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet 2001; 10: 721–33
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991; 66: 589–600
- Van der Luijt R, Khan PM, Vasen H, Van Leeuwen C, Tops C, Roest P, et al. Rapid detection of translation-terminating mutations at the adenomatous polyposis coli (APC) gene by direct protein truncation test. Genomics 1996; 20: 1–4
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 2002; 30: e57
- Mioshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, et al. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum Mol Genet 1992; 1: 229–33
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, et al. Characteristics of somatic mutations of the adenomatous polyposis gene in colorectal tumors. Cancer Res 1994; 54: 3011–20
- Huang J, Zheng S. Characteristics of APC gene mutations in colorectal tumors. Chin J Med Genet 2001; 18: 351–5
- Wallis YL, Morton DG, McKeown CM, Macdonald F. Molecular analysis of the APC gene in 205 families: Extended genotype-phenotype correlations in FAP and evidence for the role of APC amino acid changes in colorectal cancer predisposition. J Med Genet 1999; 36: 14–20
- Gismondi V, Bafico A, Biticchi R, Pedemonte S, Molina F, Heouaine A, et al. Characterization of 19 novel and six recurring APC mutations in Italian adenomatous polyposis patients, using two different mutation detection techniques. Hum Mutat 1997; 9: 370–3
- De Filippo C, Luceri C, Caderni G, Pacini M, Messerini L, Biggeri A, et al. Mutations of APC gene in human sporadic colorectal cancers. Scand J Gastroenterol 2002; 37: 1048–53
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci 1999; 96: 1603–8
- Rubinfeld B, Albert I, Porfiri E, Munemitsu S, Polakis P. Loss of β-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res 1997; 57: 4624–30