Abstract
Bisphosphonates and chemotherapy have increasingly gained favour in the treatment of metastatic hormone resistant prostate cancer. We investigated whether zoledronic acid, at a concentration found at the bone, would enhance the anti-tumour activity of docetaxel in the hormone resistant prostate cancer cell line PC-3. Cells were exposed to zoledronic acid (1 mM) in combination or in sequence with docetaxel (3 nM). Cell viability, apoptosis and markers for inhibition of the mevalonate pathway were analyzed 48 or 72 hours after drug treatment. Reduction in cell viability and increased apoptosis levels were most pronounced with single agent zoledronic acid. Western blot analysis showed an overall reduction in the proliferation marker Mini chromosome maintenance protein 2 (MCM2) and reduction in caspase-3 precursor for all drug treatments and a marked reduction in Rho A levels with single agent zoledronic acid and zoledronic acid-docetaxel sequence. This study highlights the potency of zoledronic acid, when used at concentrations similar to those found at the bone, in reducing cell viability and causing apoptosis. Clinically, these findings suggest that in patients with bone metastases due to hormone resistance prostate cancer, who are not fit enough for systemic chemotherapy, single agent zoledronic acid may have a direct effect on viability of prostate cancer epithelial cells.
Androgens play a major role in promoting the development and progression of prostate cancer. As a result, current therapy of advanced prostate cancer usually involves either androgen ablation with orchidectomy or luteinising hormone releasing hormone (LHRH) or blockade of androgen action through the androgen receptor (AR). This treatment results in improved symptoms and a reduction in the prostate specific antigen level (PSA). While this anti-androgen therapy often results in a significant response, this control typically only lasts between 18 and 36 months. During this time most patients will progress and eventually develop hormone-resistant prostate cancer (HRPC), with a median survival of approximately one year Citation[1]. Two treatment options have increasingly gained favour in HRPC in recent years: bisphosphonates, used in patients with bone metastases because of their inhibitory action on osteoclast-mediated bone resorption, and chemotherapy. Skeletal involvement is common in prostate cancer and this has stimulated research on the role of bisphosphonates in HRPC. Zoledronic acid, a third generation amino-containing bisphosphonate, has been shown to reduce the incidence of skeletal events in patients with prostate cancer in a randomized, placebo controlled trial Citation[2]. As a result, zoledronic acid is now used in patients with symptomatic bone metastases from HRPC. In addition to their action in reducing skeletal related events, it has been speculated that bisphosphonates, especially the more potent amino-containing compounds, may have a direct effect on prostate cancer epithelial cells themselves Citation[3]. The mechanism of action is believed to be inhibition of the mevalonate pathway, by preventing farnesylation and geranylgeranylation of small GTP binding proteins such as Ras, Rac and Rho, activation of caspase-3 Citation[4] and altering Bcl-2 and Bax ratios Citation[3].
The role of chemotherapy in advanced HRPC has also changed over the last few years. Palliative benefit and improvement in quality of life have been demonstrated in phase III studies comparing steroids alone versus mitoxantrone given in combination with steroids Citation[5]. Although this treatment is a recognized regimen in HRPC, the lack of improvement in survival and the low rate of PSA responses (19–33%) clearly indicated that more active combinations needed to be found. The search for more effective chemotherapy regimens for HRPC has focused mainly on taxanes (paclitaxel and docetaxel). Taxanes exert their effects by binding to microtubules of the mitotic spindle making it extremely stable and static. This promotes polymerization and arrest of the cell cycle. Docetaxel, a semi-synthetic analogue of paclitaxel, has been shown to exhibit significantly longer cellular affinity and uptake as well as slower cellular efflux than paclitaxel Citation[6], thus effectively prolonging the duration the cell is exposed to the drug. Taxanes have also been shown to play a role in the induction of apoptosis via the phosphorylation and inactivation of the anti-apoptotic protein bcl-2 Citation[7], the activation of the jun N-terminal kinase (JNK) pathway Citation[8] and the activation of the caspase signalling pathway Citation[9].
Results of in vitro studies using bisphosphonates has led to particular interest in their potential to enhance the anti-tumour activity of known chemotherapeutic agents that are commonly used in the clinical setting. Although previous studies have explored the potential interactions and combinations of the various cytotoxic drugs very little is known about how bisphosphonates interact with commonly used chemotherapeutic agents. Although the combination of docetaxel and zoledronic acid is currently under clinical investigation in a phase III randomized clinical trial, a smaller phase II study has shown the combination of weekly docetaxel and zoledronic acid is safe and associated with a 48% serum PSA response rate (reduction of 50% or more compared to baseline) Citation[10].
However, the relevance of the anti-tumour effects of bisphosphonates observed in vitro, at clinically achievable concentrations, either alone or in combination with taxanes is not well understood. Therefore, the aim of this study was to investigate the effects of zoledronic acid and docetaxel treatment, either as single agents, in combination or in sequence, on the androgen independent prostate cancer cell line PC-3 at doses clinically achievable at sites of bone metastases.
Material and methods
Cell lines
The human androgen-independent prostate carcinoma cell line PC-3, obtained from the American Type Culture Collection (Manassas, VA), was routinely cultured in 50: 50 mix of Ham's F-12 media and RPMI 1640 (Sigma-Aldrich, Dorset, UK), supplemented with 10% (v/v) foetal calf serum and 2 mM L-Glutamine (Gibco-Invitrogen, Paisley, UK). Cells were grown in a humidified atmosphere of 5% CO2 at 37°C. Cells were sub-cultured using 0.25% trypsin/EDTA (Sigma-Aldrich).
Drugs
Zoledronic acid was a gift from Novartis Pharmaceuticals (Basel, Switzerland). Docetaxel was a gift from Sanofi-Aventis Pharmaceuticals (Sanofi-Aventis, UK). A stock solution of zoledronic acid was prepared in phosphate buffered saline (PBS) and diluted using media. Docetaxel was reconstituted in ethanol and serial dilutions of the stock solution were performed using ethanol. Prior to use, stock solution was diluted to the required working concentration using cell culture media.
Analysis of cell viability
Finding the optimum dose of docetaxel
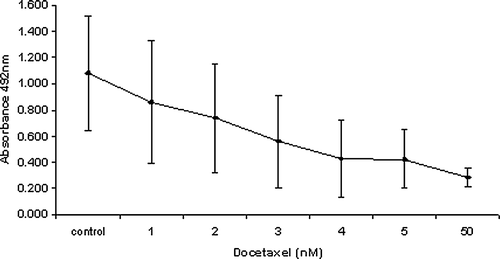
Cells were seeded at 5 000 cells/100 ul in 96 well plates and incubated at 37°C overnight to allow adherence to the plate. Cells were treated with docetaxel in the range of 1–50 nM for 24 h. Following exposure, cells were washed free of drug using PBS, fresh media was added and the cells were then left to recover for a further 48 h. Cell viability was assessed using the Cell 96® AQueous One Solution Cell Proliferation Assay (Promega, Southampton, UK) according to the manufacturers’ instructions. The One Solution assay is a colorimetric method for determining the number of viable cells; this assay utilizes a compound called MTS tetrazolium which is reduced by metabolically active cells into a colour formazan product. The amount of formazan product, measured at an absorbance of 490 nm, is directly proportional to the number of living cells in culture.
Schedule studies
Following the results from the dose finding study (), 3 nM of docetaxel was used in all subsequent experiments as it produced almost a 50% reduction in cell viability and is also a concentration achievable in the clinical setting.
Figure 1. Effect of increasing concentrations of docetaxel on PC-3 cell number following 24 h exposure and a further 48 h drug free incubation.
As we wished to investigate the proposed anti-tumour effects of zoledronic acid at the bone, a concentration of 1 mM was used following an in vivo study which reported that the concentration of zoledronic acid at sites of active bone resorption were found to be as high as 1 mM Citation[11].
Combination dosing
Cell density and plating were carried out as outlined above. Following overnight incubation, cells were exposed to docetaxel alone for 23 h followed by the addition of zoledronic acid for a further 1 h. The drugs were then removed and cells washed in PBS. Fresh culture media was added and the cells were allowed to recover for an additional 48 h before assessing cell viability.
Sequence dosing
Docetaxel then Zoledronic acid
Cells were exposed to docetaxel for 24 h. Following exposure, docetaxel was removed and the cells washed free of any residual drug using PBS. Zoledronic acid was then added for an additional 1 h. After zoledronic acid exposure, the drug was removed and cells were once again washed free of residual drug with PBS. Fresh media was added and the cells further incubated for a period of 48 h before cell viability was assessed.
Zoledronic acid then Docetaxel
Zoledronic acid was added to the cells for 1 h and then removed by washing cells in PBS. Docetaxel was then added for an additional 24 h. Following docetaxel exposure, the drug was removed, cells were washed free of residual drug with PBS and fresh media was added to allow cells to recover for a further 48 h. Cell viability was then assessed.
For combination and sequence studies, PC-3 cells were also incubated in drug free media (negative control), docetaxel alone and zoledronic acid alone (positive control). Six replicates per treatment were carried out. Wells for each replicate were randomly assigned in order to minimize intra-plate variability, and the experiments were repeated three times. It has been established that zoledronic acid acts as a chelating agent of calcium in cell culture media; PC-3 cells were therefore also incubated under identical experimental conditions with 1 mM ethylenediaminetetraacetic acid (EDTA), which chelates calcium, in order to control for the effect of calcium chelation on cell viability.
Apoptosis detection
As no single parameter can define apoptosis in all systems, three methods were performed to assess if zoledronic acid and/or docetaxel induced reduction in cell viability was through an apoptotic process.
Distinction between apoptosis and necrosis by flow cytometry
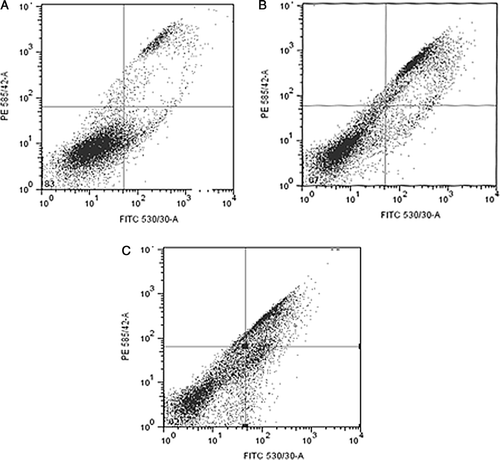
1 mM zoledronic acid, although clinically achievable at sites of active bone resorption, is a concentration at least 10-fold higher than has previously been used when exposing prostate cancer cells to a bisphosphonate in vitro. To determine the amount of programmed cell death compared to non specific cell death at 1 mM zoledronic acid, apoptosis and necrosis were assessed using the Vybrant apoptosis assay kit 4 (Molecular Probes, Invitrogen Ltd, Paisley, UK). Prostate cancer cells were exposed to 1 mM zoledronic acid for 1 h and 5 µM camptothecin for 4 h (positive control), washed free of drug and incubated in drug free media until a total of 72 h, from the start of dosing. Spontaneous cell death in cells not exposed to drugs served as a negative control. Adherent and floating cells were harvested, centrifuged and washed in PBS. Approximately 1×106 cells were resuspended in PBS and stained with 1 µl YO-PRO-1 to detect early apoptosis and 1 µl propidium iodide to detect necrosis. Samples were analyzed using a FACSaria flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) at a wavelength of 488 nm, measuring the fluorescence emission at 530 nm and >575 nm. The experiment was carried out in triplicate and the average data was used to construct dot plots in log scale to identify live, apoptotic and necrotic populations.
Measurement of apoptosis by ELISA
Cells were seeded at 5×104 in 6-well plates. Zoledronic acid and docetaxel were administered as single agents, in combination or sequence identical to that indicated for cell viability assessment. Cell lysates were then analyzed for histone-associated DNA fragments (mono- and oligonucleosomes) using the Cell Death Detection ELISA assay (Roche Diagnostics, Mannheim, Germany). This assay uses mouse monoclonal antibodies directed against DNA and histones to determine the number of mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates. Absorbance at 405 nm is directly proportional to the number of mono- and oligonucleosomes.
Investigation of apoptosis by western blot analysis
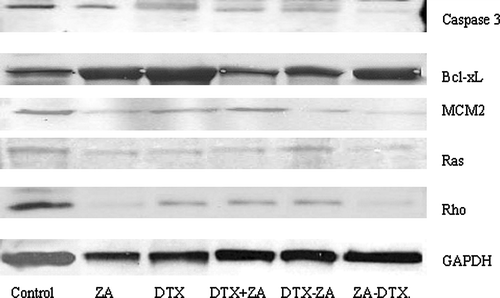
Total protein was extracted using the TRIzol method (Sigma). 50 µg of protein was run on either 7% tris-acetate or 12% bis-tris PAGE gels (Bio-Rad Laboratories, Hemel Hempstead, UK), depending on the size of the protein of interest. Proteins were transferred onto nitrocellulose membrane (Amersham Pharmacia Biotech). The membranes were blocked with 5% non-fat dry milk in tween/TBS to inhibit non specific binding and incubated overnight at 4°C with rabbit polyclonal Bcl-xL (1:00) and caspase 3 (1:100) as a measure of apoptosis.
In addition, goat polyclonal MCM2 (1:200) was used as a measure of proliferation, rabbit polyclonal pan Ras (1:50) to show effects of farnesylation and Rho A (1:100), to indicate effects on geranylgeranylation were also investigated. GAPDH (1:600) was used as a control for protein loading. Membranes were washed free of primary antibody and incubated with horseradish peroxidase conjugated secondary anti-goat/anti-rabbit antibodies (1:1000) for 1 h at room temperature. Proteins were visualized using chemiluminescene luminol reagent (all Santa Cruz Biotechnology, Santa Cruz, California, USA).
Statistical analysis
To test the assumption that data were normally distributed, Normal plots were performed for each group. Kolmogorov-Smirnov statistics resulted in p-values >0.05 indicating data was normally distributed. Analysis was, therefore, carried out using the parametric ANOVA (multiple group comparisons) and unpaired, two-tailed Student t-test (pair-wise comparisons). A p-value <0.05 was considered significant for the ANOVA test, while p-values <0.003 was considered significant for the t-tests after the Bonferroni procedure was applied. The Bonferroni procedure is used for multiple comparisons and corrects the p-value accordingly by dividing the desired p-value by the number of comparisons made (i.e. 15 pair wise comparisons were made for cell viability, ELISA and cytometric analysis. Therefore 0.05/15 gives a corrected p-value 0.003). This makes for a more robust analysis of the data and reduces the possibility of false positives. Results are expressed as the mean (3 separate experiments combined)±SD.
Results
Effect of zoledronic acid and docetaxel on cell viability
The percentage of viable cells remaining after drug treatment was determined by comparison with control, untreated, cells and showed both zoledronic acid and docetaxel significantly reduced the number of viable prostate cells (p < 0.0001), compared to control cells. Further analysis showed the reduction in cell viability was significantly more pronounced with exposure to single agent zoledronic acid compared to single agent docetaxel (p < 0.0001). Additionally, treatment with zoledronic acid as a single agent was more effective than in combination with docetaxel (p = 0.0008). Single agent zoledronic acid was also as effective as sequence dosing. Sequence dosing produced a greater reduction in cell viability than drug combination (p < 0.0001) but there was no significant difference regarding the order of drug sequence (, ). Cells treated with EDTA did not produce a significant reduction in cell viability when compared to controls.
Figure 2. PC-3 cells treated with zoledronic acid (1 mM) for 1 h, docetaxel (3 nM) for 24 h, media alone (control) and either drugs in combination or in different sequence context. All treatment produced a significant reduction (p ≤ 0.0001) in cell viability compared to controls.
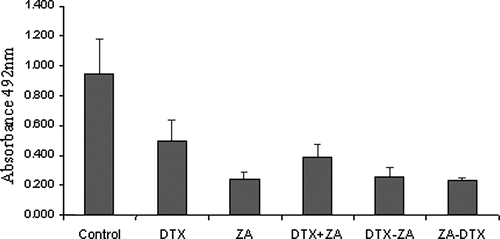
Table I. Statistical analysis for cell viability study.
Levels of apoptosis and necrosis following zoledronic acid exposure
Having established that zoledronic acid and docetaxel reduced PC-3 cell viability, we sought to determine if necrosis contributed significantly to this reduction. As exposure to docetaxel was carried out at a concentration that is within the range widely accepted in the published literature (and its necrotic and apoptotic effects already studied Citation[12]) we focused only on the apoptotic and necrotic potential of zoledronic acid.
Cells that stained positive for YO-PRO-1 but negative for propidium iodide were scored as apoptotic, while cells with high levels of staining for both YO-PRO-1 and propidium iodide were scored as necrotic. The percentages of cells that underwent spontaneous, zoledronic acid or camptothecin induced apoptosis and/or necrosis are shown in . Cytometric dot plots revealed the number of viable cells did not decrease with camptothecin treatment but there was a 50% reduction in cell viability after zoledronic acid exposure compared to control cells. An increase in the level of necrosis was observed by a rise of 54% above control levels in camptothecin treated cells and a 67.5% increase following zoledronic acid exposure. However, this increase in drug induced necrosis was not significant at the corrected p < 0.003 level. Low levels of apoptosis were seen in all sample groups and no significant difference observed when levels were compared between groups. There was also no significant difference between the levels of apoptosis or necrosis in all samples. Thus, the reduction in cell viability, in particular the reduction induced by zoledronic acid, was the result of both apoptotic and necrotic events with neither one making a significant contribution.
Figure 3. Cytometric dot plots of forward and side scatter parameters (x-axis, 530 nm: apoptosis; y-axis, 585 nm: necrosis). A. Control cells, B. Camptothecin (5 µM) treated cells and C. Zoledronic acid treated cells (1 mM). The upper right quadrant shows% necrotic cells (YO-PRO-1 and PI positive), lower left quadrant shows% viable cells (YO-PRO-1 and PI negative) and lower right quadrant shows% apoptotic cells (YO-PRO-1 positive, PI negative).
Apoptosis analysis using ELISA
Cell death analysis, on all dosing regimens, was carried out using a one-step colorimetric immunoassay for the enrichment of mono- and oligonucleosomes. Measuring the levels of DNA fragmentation allowed a further and more specific differentiation and quantification of apoptosis by removing the necrotic faction of the lysate. Data from the ELISA assay () showed both single agent zoledronic acid and docetaxel increased the level of apoptosis in treated cells when compared to non-treated controls (p < 0.0001). Single agent zoledronic acid again showed a significantly higher increase in nucleosomal levels than those of docetaxel treated cells and combination treatment (p < 0.0001). However ELISA results contradicted the cell viability data with regards to the sequencing regimens. In the cell viability study, no significant difference was found between sequencing regimens yet the ELISA analysis showed zoledronic acid followed by docetaxel produce a significantly higher level of cytoplasmic nucleosomes than docetaxel administered before zoledronic acid (p < 0.0001). There was also no significant difference in nucleosomal levels when drugs were given in combination compared to docetaxel→zoledronic acid (p > 0.003, see for statistical comparisons).
Figure 4. Analysis of drug induced cell death. Mono- and oligonucleosomes, in cytoplasmic fractions of cell lysates, were determined by a photometric enzyme immunoassay.
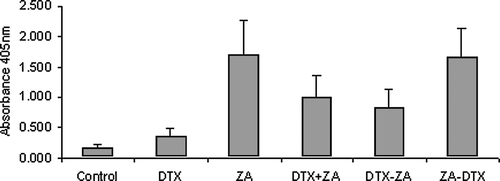
Table II. Statistical analysis for apoptosis analysis.
Identification of changes in protein expression of proliferation and apoptosis markers after zoledronic acid and docetaxel treatment
Zoledronic acid has been shown to exert its anti-tumour effects by inhibiting farnesyl diphosphate synthase, which leads to downstream inhibition of signalling proteins such as Ras and Rho and activates caspase-3. Docetaxel, meanwhile, is believed to down regulate the expression of the anti-apoptotic protein Bcl-xL. Western blot analysis () revealed protein expression for Rho A in zoledronic acid and the sequence zoledronic acid → docetaxel treated cells were markedly reduced in comparison to controls and the other drug treated samples. Ras protein levels, however, did not show any considerable change in any of the samples. Bcl-xL levels were also unchanged across all samples. Caspase-3 levels, of the 32kda precursor, were decreased in all drug treated samples suggesting cleavage of the inactive form into its two active subunits. Interestingly, the caspase-3 levels for single agent zoledronic acid and the sequence zoledronic acid→docetaxel were different even though there had been no significant difference in treatment effect with regards to cell viability or apoptosis. MCM2, which was included as an additional marker for proliferation, highlighted the observation that drug treated cells showed an overall lower expression of proliferation, with zoledronic acid and zoledronic acid→docetaxel treated cells showing the greatest reduction in MCM2 protein levels.
Discussion
From this study, we have shown that treatment of the prostate cancer cell line PC-3 with 1 mM zoledronic acid produced a significant reduction in cell viability and increase in apoptosis when compared to untreated control cells or cells treated with single agent docetaxel at 3 nM. In addition, single agent zoledronic acid was also more effective at inducing apoptosis than when in combination with docetaxel or when administered after docetaxel.
Several groups have studied the anti-proliferative and/or pro-apoptotic effects of zoledronic acid on breast Citation[13], and prostate Citation[14–16] cancer cell lines. However, these studies report contradictory data. For prostate cancer in particular, some studies claim the anti-tumour effects of zoledronic acid is the result of cytostasis rather than cell death Citation[15] whereas others report cell reduction via an apoptotic pathway Citation[5]. In the majority of these investigations, cells were incubated for extensive periods (up to 6 days) at various concentrations (from 10–200 µM). These parameters do not reflect the clinical setting as they neither reflect peak serum concentrations, concentrations reported to be found in the bone or the duration of exposure. Peak plasma concentrations of zoledronic acid range from 1–2 µM following a 4 mg infusion Citation[17] and are only maintained for a short period before avidly binding to bone or being excreted unchanged in the urine. An in vivo study conducted by Sato et al. Citation[11] reported that local concentrations of bisphosphonates, released at sites of active bone resorption, could reach levels as high as 1 mM. Therefore, in patients with metastases to the bone it could be assumed that the close proximity of tumour cells to osteoclasts would cause them to be exposed to high levels of bisphosphonate following osteolysis. We dosed PC-3 cells, derived from a prostate tumour that had metastasized to the bone, with 1 mM zoledronic acid for 1 h. Emulating the concentrations believed to be achieved at the bone resulted in a significant reduction in cell viability. It should be noted that zolederonic acid has the ability to chelate calcium. However studies with EDTA, which also chelates calcium, did not result in a significant reduction in viable cells. This suggests that the significant decrease in cell viability following zoledronic acid treatment must be due to other factors.
Flow cytometric analysis to elucidate whether this reduction was due mainly to apoptosis or necrosis revealed no significant difference between either manners of cell death even though a significant increase in apoptotic levels was detected by ELISA and confirmed with western blotting using the apoptotic marker cleaved caspase 3. This discrepancy could be explained by the sensitivity of the assays and the end points which they detect. It is well known that apoptosis is mediated by the activation of a multitude of enzymes, some of which are specific to certain cell types/tissues, cell differentiation and apoptotic stimuli. Thus, no single technique can be used to distinguish apoptosis. In vitro, for example, it has been shown that in the absence of phagocytes, apoptotic cells can undergo a secondary non-specific degradation allowing them to take up dyes that would normally only stain necrotic cells Citation[18]. Cells in these experiments were subjected to different experimental conditions over 72 h before apoptosis was assessed yet YO-PRO-1 used in the flow analysis is a marker of early apoptosis. Thus, secondary degradation, also known as secondary necrosis, could be the explanation for the lack of apoptosis seen in the zoledronic acid treated cells when analyzed using flow cytometry.
To date, docetaxel is the only licensed taxane for use in HRPC. PC-3 cells were exposed to 3 nM docetaxel, a clinically achievable concentration. We found single agent zoledronic acid to be significantly more effective than docetaxel at reducing cell viability and inducing apoptosis. In agreement with our finding, Tantivejkul et al. Citation[19], showed exposure of DU145 cells to 100 nM of docetaxel only induced apoptosis in 20% of cells and yet this cell line is reported as being more sensitive to docetaxel than PC-3 cells Citation[20]. An explanation for this diminished cytotoxic effect may be provided by the discovery that prostate cells express bone resorbing factors such as parathyroid hormone-related protein (PTHrP), interleukin 1 (IL-1) and interleukin 6 (IL-6). These bone microenvironment- related growth factors are believed to aid survival of metastatic prostate cancer cells by inhibiting chemotherapy induced apoptosis Citation[21] and have been shown to inhibit doxorubicin-induced, but not zoledronic acid-induced, apoptosis of PC-3 cells Citation[22].
Synergism between bisphosphonates and chemotherapeutic agents has been reported in the treatment of human breast cancer cells Citation[17] and small cell lung cancer Citation[23]. More recently an in vitro investigation of zoledronic acid combined with docetaxel has been reported for prostate cancer Citation[20], although not at concentrations achievable in the clinic. Consistent with previous studies, we found that PC-3 cells, when exposed to docetaxel, in combination with zoledronic acid, produced a significant reduction in cell viability and increase in apoptosis compared to controls. However, although cell viability studies revealed drugs administered in sequence to be significantly more effective than combination (but no difference with respect to the order), apoptosis studies of sequence effect showed zoledronic acid administered before docetaxel to be the most potent sequencing regimen. It is unclear why these observations are inconsistent. They may reflect a docetaxel-induced delay in apoptosis due to a lack of Bcl-xL down regulation or doses required to induce apoptosis may need to be higher than doses needed to inhibit growth and decrease cell viability Citation[14], Citation[16]. Pair-wise comparisons between single agent zoledronic acid and combination and sequence dosing revealed single agent zoledronic acid to be as or more effective than any other schedule. Thus, it may be argued that concentrations of zoledronic acid at levels similar to those found at the bone are so potent that chemotherapy provides little additional benefit.
Minichromosome maintenance (MCM) proteins (MCM2-7) are part of the pre-replication complex required for DNA replication. A recent study Citation[24] suggested MCM proteins maybe useful molecular markers for cell proliferation while prostate cancer patients with high MCM2 expression have been shown to exhibit shorter disease free survival Citation[25]. Western blot analysis of MCM2 revealed cells exposed to either zoledronic acid or docetaxel had lower levels of MCM2 protein expression compared to control levels with the most pronounced reduction in single agent zoledronic acid and zoledronic acid followed by docetaxel. It maybe suggested that MCM2 could be used, not only as a marker of proliferation but also to indicate response to chemotherapy. Analysis of total Ras protein levels from all treatment groups did not differ with respect to control levels. However total Rho A protein levels were markedly reduced in the zoledronic acid and the sequence zoledronic acid followed by docetaxel. Although these findings are not a direct measure of reduced protein prenylation, they may help support the current hypothesis that zoledronic acid predominately exerts its effect through the mevalonate pathway by preventing geranylgeranylation and hence down regulation of Rho. In addition, the caspase-3 analysis, could not completely explain the mechanisms of apoptosis seen, particularly concerning single agent zoledronic acid, suggesting additional apoptotic pathways must be involved.
Conclusion
Our data has demonstrated that zoledronic acid is either as potent as, or superior to any of the cytotoxic regimens employed in this study. We have reported significant decreases in cell viability and increases in apoptosis levels using a concentration reportedly found at sites of active bone resorption, a concentration that has not previously been investigated in vitro. We also conclude that a more effective schedule appears to be zoledronic acid (bisphosphonate) administered prior to the chemotherapeutic drug. Since these data have been generated using only one cancerous cell line, we need to verify these findings in other prostate cell lines, organ culture models and in vivo and fully elucidate the molecular mechanisms and functional pathways through which these drugs are causing their effects. We also plan to investigate whether the effects of zoledronic acid or other bisphosphonates with or without taxanes, vary with the lower concentrations of potent bisphosphonates found in the serum, which might have implications for therapeutic effects on visceral metastases.
Clinically these findings could suggest that in patients with bone metastases due to HRPC who are not fit enough for systemic chemotherapy, single agent zoledronic acid may have a direct effect on metastatic prostate cancer epithelial cells in addition to the proven reduction in skeletal morbidity by its effects on osteoclast function.
Acknowledgements
We wish to thank Mark Roberts for his technical help and expertise. This project received funding from Novartis and Sanofi-Aventis.
References
- Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol 2002; 20: 3972–82
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–68
- Coxon JP, Oades GM, Kirby RS, Colston KW. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int 2004; 94: 164–70
- Senaratne SG, Mansi JL, Colston KW. The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br J Cancer 2002; 86: 1479–86
- Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol 1999; 17: 1654–63
- Riou J. Cellular uptake and efflux of docetaxel (Taxotere) and paclitaxel (Taxol) in P388 cell line. Proc Am Assoc Cancer Res 1994; 35: 385
- Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res 1997; 57: 229–33
- Wang Q, Wieder R. All-trans retinoic acid potentiates Taxotere-induced cell death mediated by Jun N-terminal kinase in breast cancer cells. Oncogene 2004; 23: 426–33
- Muenchen HJ, Poncza PJ, Pienta KJ. Different docetaxel-induced apoptotic pathways are present in prostate cancer cell lines LNCaP and PC-3. Urology 2001; 57: 366–70
- Bertelli G, Heouaine A, Arena G, et al. Weekly docetaxel and zoledronic acid every 4 weeks in hormone-refractory prostate cancer patients. Cancer Chemother Pharmacol 2005; 57: 46–51
- Sato M, Grasser W, Endo N, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 1991; 88: 2095–105
- Suzuki A, Kawabata T, Kato M. Necessity of interleukin-1beta converting enzyme cascade in taxotere-initiated death signaling. Eur J Pharmacol 1998; 343: 87–92
- Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. Br J Cancer 2001; 84: 1126–34
- Oades GM, Senaratne SG, Clarke IA, Kirby RS, Colston KW. Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol 2003; 170: 246–52
- Dumon, JC, Journe, F, Kheddoumi, N, Lagneaux, L, Body, JJ. Cytostatic and apoptotic effects of bisphosphonates on prostate cancer cells. Eur Urol 2004;45:521–8; Discussion 8–9.
- Nogawa M, Yuasa T, Kimura S, et al. Zoledronic acid mediates Ras-independent growth inhibition of prostate cancer cells. Oncol Res 2005; 15: 1–9
- Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, Coleman RE, Holen I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer 2005; 113: 364–71
- Gomez-Lechon MJ, O'Connor E, Castell JV, Jover R. Sensitive markers used to identify compounds that trigger apoptosis in cultured hepatocytes. Toxicol Sci 2002; 65: 299–308
- Tantivejkul K, Loberg RD, Mawocha SC, et al. PAR1-mediated NFkappaB activation promotes survival of prostate cancer cells through a Bcl-xL-dependent mechanism. J Cell Biochem 2005; 96: 641–52
- Ullen A, Lennartsson L, Harmenberg U, et al. Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol 2005; 44: 644–50
- Reyes-Moreno C, Sourla A, Choki I, Doillon C, Koutsilieris M. Osteoblast-derived survival factors protect PC-3 human prostate cancer cells from adriamycin apoptosis. Urology 1998; 52: 341–7
- Tenta R, Tiblalexi D, Sotiriou E, Lembessis P, Manoussakis M, Koutsilieris M. Bone microenvironment-related growth factors modulate differentially the anticancer actions of zoledronic acid and doxorubicin on PC-3 prostate cancer cells. Prostate 2004; 59: 120–31
- Matsumoto S, Kimura S, Segawa H, et al. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer 2005; 47: 31–9
- Padmanabhan V, Callas P, Philips G, Trainer TD, Beatty BG. DNA replication regulation protein Mcm7 as a marker of proliferation in prostate cancer. J Clin Pathol 2004; 57: 1057–62
- Meng MV, Grossfeld GD, Williams GH, et al. Minichromosome maintenance protein 2 expression in prostate: Characterization and association with outcome after therapy for cancer. Clin Cancer Res 2001; 7: 2712–8