Abstract
The aim of this study was to evaluate the outcome for gastric cancer patients treated at a medium sized Norwegian hospital. The medical journals of all 356 patients with gastric cancer treated at Levanger Hospital from 1980 to 2004 were retrospectively analysed. Follow-up with regard to survival was complete. The Department of Surgery had treated 277 patients (78%). The resection rate of patients admitted to the Department of Surgery was 56% (154/277), and the total resection rate was 43% (154/356). R0 resection was done in 97 patients (27%), R1 resection in 16 (4%), palliative R2 resection in 41 (12%), other palliative procedures in 59 (17%), and only palliative care was given for 143 (40%) patients. The 30-days postoperative mortality was 2.7% (3/113) after R0 and R1 resections, 4.9% (2/41) after R2 resections, and 24% (14/59) after other palliative procedures. After R0 resections, the estimated overall 5-year survival was 39% (95% C.I. 29–49). After R1 and R2 resections, none survived 5 years and the estimated overall 2-year survival was 12% (95% C.I. 0–27%) and 2% (95% C.I. 0–7%), respectively. Estimated overall 5-year survival was closely related to stage: 91% (95% C.I. 74–100) in stage 1A, 64% (95% C.I. 53–74) in stage 1B, 27% (95% C.I. 10–44) in stage II, 18% (95% C.I. 4–32) in stage IIIA, and none in stages IIIB and IV. Dysphagia, fatigue, weight loss, palpable tumour, ascites and anaemia were related to a bad prognosis. Dyspepsia, vomiting and hematemesis were not related to the prognosis. Symptoms duration > 6 months were related to a better prognosis than short duration of symptoms < 2 months. The results from this hospital are in accordance with previous reports from the Western world.
Worldwide, gastric cancer has been the second most common cause of cancer death. The incidence has decreased in the Western world during the second half of the twentieth century. Though due to increasing age and growth in the population, the absolute number of new cases has not shown the same decline. The age-adjusted incidence in males and females in Norway has changed from 38 and 22 per 100 000 in 1956 to 8.1 and 4.4 in 2004. During the five-year period 1957 to 1961 the mean number of new cases per year was 1 424 whereas the number in 2004 was reduced to 567 Citation[1].
In Japan, mass screening programmes have lead to detection of gastric cancer in earlier stages Citation[2]. In contrast to this, most cases in Western countries are detected in advanced stages, which have a more severe prognosis. The different survival rates observed within Europe have been mainly attributed to variation in stage distribution and the diversity of populations at the different hospital levels Citation[3–5].
The aim of the present population-based study was to evaluate the results from a Norwegian county hospital and to compare them with results published elsewhere. This evaluation and comparison focused on short and long-term outcome, which were defined as 30-days postoperative mortality and 5-year survival. Moreover, we wanted to see if specific types of symptoms and signs and the duration of the symptoms were related to long-term survival.
Patients and methods
Patients
The records of all consecutive patients treated for gastric cancer at Levanger Hospital from 1980 to 2004 were reviewed. This hospital serves a well defined geographical area with 87 000 inhabitants in the middle of Norway. The patients were identified from the hospital patient registry system, and a complete cohort was confirmed using data registered at The Cancer Registry of Norway. From the 373 patients thus identified, we excluded eight patients who were referred to and operated on at the University Hospital of Trondheim, eight who did not have adenocarcinomas (five leiomyosarcomas, two lymphomas, and one gastrointestinal stromal tumour) and one with a missing record. The diagnosis was based on histological examination of the removed surgical specimen in 152 cases, endoscopic biopsy in 173, tissue from autopsy in 17 and only clinical and radiological examination in 14 patients.
The present study was based on the remaining 356 patients, of which 222 were male and 134 were female with a median age of 76.4 years (range 22.5–94.9). The Department of Surgery treated 277 patients, the Department of Medicine treated 63, and 16 were treated as outpatients. The main location of the tumours were in the cardia in 80, fundus in 36, antrum or corpus in 147, pylorus in 28, diffuse in the entire stomach (linitis plastica) in 30, and stump cancer in 35. The tumours were in more than one defined region in 82 of these patients (23%). Anaesthesia risk due to accompanying diseases was classified according to the guidelines of the American Society of Anaesthesiologists Citation[6].The duration of symptoms was defined as the time from the first symptom until the diagnoses was made. The duration was divided into three intervals: < 2 months, 2–6 months and >6 months.
The type of treatment is shown in . The resection rate was 43% (154/356). The 154 resections were distributed in 97 R0 resections, 16 R1 resections, and 41 palliative R2 resections. In 143 of all cases no surgical or endoscopic treatment was performed: in 69 cases the tumour was considered unresectable, in 50 because of severe coexisting diseases, 20 patients chose not to undergo an operation, and in four cases the diagnosis was made post mortem.
Table I. 30-days mortality related to treatment.
Adjuvant chemotherapy was not used. In the palliative setting, 66 patients were treated with chemotherapy; 34 had 5-FU treatment, 18 FAM (5-fluorouracil), doxorubicin and mitomycin), 11 ELF (etoposid, leucovorine and 5-fluorouracil) and 3 FLOX (5-fluorouracil, leucovorine and oxaliplatin). ELF was used from 1998 and FLOX from 2004.
The term postoperative mortality is defined in different ways in different investigations. To be able to compare the results from the present study to previous publications, we specified postoperative mortality in three ways: (1) death within 30 days after surgery, whether inside or outside the hospital, (2) death during the hospital stay, regardless of when death occurred, or (3) death during the hospital stay or death outside the hospital within 30 days after surgery. The 25-year period was divided into 5-year groups to be able to evaluate trends in postoperative mortality and long-term survival. None were lost to follow-up with regard to survival. In April 2006, 333 were dead and 23 were still alive. The median time of follow-up for patients still being alive was 8.4 years (range 1.5–25.9).
Surgical principles and postoperative routines
Diagnostic routines and treatment was supervised by a specialist in gastrointestinal surgery from 1980, three specialists from 1988. The surgical treatment was performed by a specialist in 61% of the cases and assisted by a specialist in 79% of the cases when a non-specialist was operating. In general, a D1 resection entailed the removal of lymph nodes from the left and right gastric arteries and from the celiac axis. The lesser and greater omentum were completely removed, but the upper part of the greater omentum was left in distal resections (D0). Formal D2 resection was rarely performed. In all resections involving the oesophagus, the anastomosis was tested by a roentgen examination seven days after surgery before oral intake was allowed.
The surgeon did not dissect out the lymph nodes from the different lymph node stations before delivering the surgical specimen to the pathologist. The median number of lymph nodes examined and reported by the pathologists in R0 and R1 resections was 7 (range 0–30).
Statistical analyses
In-hospital mortality was analysed using logistic regression. Kaplan-Meier survivor functions, with corresponding estimates and 95% confidence intervals for survival, were calculated. The survivor functions were compared using the Log-rank test. A Cox's proportional hazards model was fitted to the data. Backwards elimination was used for variable selection in the regression analyses. All tests were two-sided, and p-values <0.05 were considered significant. The analyses were performed using SPSS 13.0.
Results
30-days mortality
The 30-days mortality after R0 resections was 2.1% (2/97). When R0 and R1 resections were combined, the mortality was 2.6% (3/113). shows the 30-days mortality and total in-hospital mortality related to the different types of treatment. The total in-hospital mortality was 8.0% (9/113), 9.7% when R0 and R1 resections were combined and 14.6% (6/41) in R2 resections. In-hospital death or death within 30 days after surgery was 3.1% (3/97) in R0 resections, 38% (6/16) in R1 resections, and 17% (7/41) in R2 resections.
In a multivariate logistic regression analysis the following factors were analyzed in relation to in-hospital mortality: age, 5-year periods of treatment (5 periods), accompanying diseases (ASA-score), and type of treatment. Type of treatment and ASA-score were independently associated with death during the primary hospital stay, see . The median postoperative stay was 11 days (range 3–90).
Table II. Adjusted odds ratio for risk of in-hospital mortality for different risk factors (logistic regression analysis of 356 patients)
Postoperative complications
One or more complications were noted in 78 patients (38%). Of seven patients with postoperative bleeding, three had to be reoperated to stop the bleeding, and none of these patients died postoperatively. One of three patients with wound-rupture died due to massive postoperative complications. Four of 12 patients who developed an anastomotic leakage had to be reoperated, whereas the other eight were conservatively treated. Three with anastomotic leakage died during their hospital stay, one of them had been reoperated for her condition. After the 154 resections, the in-hospital causes of death for 15 patients were as follows: cardiovascular complications in five patients, infective complications in four patients, and advanced cancer in six patients.
Long-term outcome
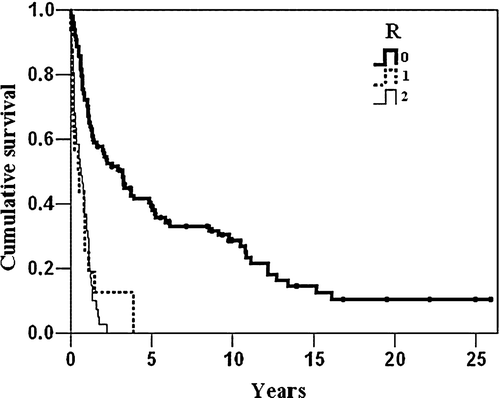
The overall 5-year survival after R0 resections was 39% (95% C.I. 29–49). None survived 5 years after R1 and R2 resections, and the estimated 2-year survival was 12% (95% C.I. 0–27%) and 2% (95% C.I. 0–7%), respectively. shows the overall survival distributed by R-type of resection. Patients with tumours located at the cardia had an estimated 5-year overall survival rate of 19% (95% C.I 0–38) after R0 resections, while the corresponding survival rate for those with non-cardia tumours was 44% (95% C.I 32–55), p = 0.05 (log rank test).
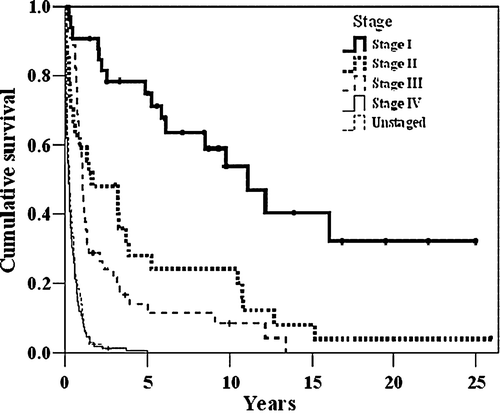
The survival rate was distributed by the four main stages as shown in . The estimated 5-year overall survival rate in stage IA was 91% (95% C.I. 74–100), in stage IB 64% (95% C.I. 53–74), in stage II 27% (95% C.I. 10–44), in stage IIIA 18% (95% C.I. 4–32), and in stages IIIB and IV 0%.In a multivariate Cox's regression analysis, the following variables were tested with regard to long-term survival: age, 5-year-period of treatment (five periods), tumour located at the cardia or not, R-type of resection, histological grade, and stage. Stage and R-type of resection were independently associated with survival, see .
Table III. Survival analysis using Cox's Proportional Hazards Model (analysis of 356 patients)
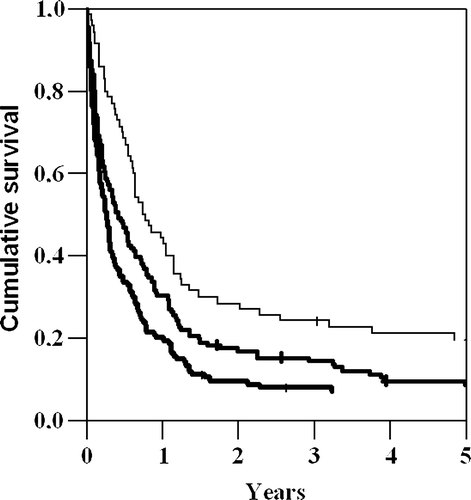
The survival rate was distributed by the duration of symptoms as shown in . Patients with longest duration of symptoms at admission, had longer survival than patients with shorter duration of symptoms. The difference in survival between the groups was significant (p < 0.001, log rank test). In patients with symptoms’ duration of less than 2 months, 6% (8/134) were diagnosed with stage I disease compared to 20% (14/70) when the symptoms had lasted for more than 6 months. Even when the patients with stage I disease were excluded from the analysis, patients with duration of symptoms >6 months survived significantly longer than those with shorter symptoms’ duration.
shows the results of a Cox's regression analysis where the following symptoms and signs and duration of symptoms at presentation were analysed with regard to long-term survival: Dysphagia, vomiting, dyspepsia, fatigue, hematemesis, weight loss, palpable abdominal tumor, ascites, anaemia defined as haemoglobin levels below 10 g/100 ml and duration of symptoms (three time intervals). All these factors were independently related to survival, except dyspepsia, vomiting and hematemesis. Long duration of symptoms was related to a better survival than short duration of symptoms.
Table IV. Survival analysis using Cox's Proportional Hazards Model (analysis of 354 patients)
Discussion
The main findings of this study from a medium sized hospital were low postoperative 30-days mortality and long-term survival that was well within the range of previous western publications. Thirty days postoperative mortality in most studies varies between 2.3% and 8%, while total in-hospital mortality is higher. In the surgical literature, 30 days postoperative mortality is a frequently used measurement when determining the death rate after an operation. However, not all studies define what is meant by postoperative mortality, making it difficult to compare the results. gives an overview of short and long-term mortality reported from various countries. In the randomized Dutch Gastric Cancer Trial, postoperative mortality included in-hospital death or death within 30 days after surgery Citation[7]. D1 resections resulted in a lower mortality rate than D2 resections, 4% and 10% respectively. In selected patient groups, as low postoperative mortality as 0.6–0.8% has been published from South-Korea and Japan Citation[16], Citation[17].
Table V. Post-operative mortality and 5-year survival in different studies.
Long-term survival is closely correlated to the stage distribution of the patients included in different studies. Total 5-year survival in the different studies presented in range from 19% to 62%. The lowest survival rate was reported in patients with cardia cancer treated in Sweden during the period 1974 to 1982 Citation[10], while the highest survival rate was reported in a Japanese study of patients 75 years or younger Citation[17]. In another study from Japan Citation[20], 2 605 healthy subjects were followed-up with repeated health checks every year in a period of 10 years from 1988 to 1998. Gastric cancer was detected in 76 subjects who had a mean age of 67 years. After surgery, the 5-year survival rate was 100% for early gastric cancer limited to the mucosa or submucosa, 41% for gastric cancer confined to the subserosal layer, and 23% for more advanced cancer.
Though retrospective studies are useful tools to define and understand clinical issues, they have their weaknesses. One weakness with the present study is the low number of lymph nodes that were examined. Because less than seven lymph nodes were examined in half of the patients who underwent R0 and R1 resection, understaging may have occurred. Patients with insufficient number of examined lymph nodes may have been placed in a stage with a higher expected survival rate than they belonged in; thereby reducing the survival rates for the stage they incorrectly were allocated into. A higher number could have been obtained if the surgeon had separately delivered the lymph nodes from the different lymph node stations. Delivering the whole surgical specimen in one block to the Department of pathology, as was done during the study period, often results in a lower number of examined lymph nodes. Another weakness of this study was that the operative report was not always specific concerning whether a macroscopic complete removal of the tumour had been done. Some of the patients that were placed in the R1 resection group may belong to the R2 group. This is noteworthy as the outcome for the R1 patients more closely resembles that of the R2 group.
A strength of this study is that all patients diagnosed with gastric cancer in the area served by this hospital were included, and as such shows a realistic view of the stage distribution and accompanying prognosis in an unselected population. The only endoscopic and roentgen units in the area are located inside the hospital. The present study underlines the seriousness of this type of cancer as 40% were not given any surgical treatment and only 27% of all patients could undergo a R0 resection. The aggressiveness of this cancer is reflected in the very short survival rate in the majority of patients, except for those having R0 resection.
Studies from university hospitals typically report of 70% total resection rate, which is much higher than in the present report, 43%. Most reports on gastric cancer treatment from large hospitals are based on selected patient groups, partly referred for surgery from smaller hospitals.
It seems obvious that the sooner a patient is treated for cancer, the better the prognosis will be. On the other hand, this study showed that long symptoms’ duration was associated with more frequent stage I cancer at presentation and a better prognosis. One possible explanation is that mild symptoms, that do not alarm the patient or his doctor, might be associated with a cancer disease with less aggressive behaviour and slower progress. Stephens et al. Citation[21] showed that specific alarm symptoms in gastric cancer were associated with a bad prognosis, and that the median delay from onset of symptoms to definitive diagnosis was twice as long for patients without alarm symptoms (24 weeks) than for those with alarm symptoms (12 weeks). This is an indirect evidence of the relationship between long duration of symptoms and favourable prognosis, but their study did not directly analyze the relationship between delays in diagnosis and survival. In another study of young patients with gastric cancer, Maconi et al. Citation[22] found that in patients without alarm symptoms, a 6-month delay in diagnosis did not affect survival, but seemed to be related to a better outcome. The duration of symptoms is an uncertain variable in prospective as well as retrospective studies. We therefore used wide time intervals. The results have to be used carefully and do not at all mean that a delay of diagnosis is unimportant.
A major challenge in the Western world is to detect gastric cancer in earlier stages. Because of a much lower incidence than in Japan, screening for gastric cancer by endoscopy or barium meal examinations has not been advised. To increase early stage detection of gastric cancer, more simple screening methods with high sensitivity and specificity must be developed. No serum marker has yet proven helpful as a screening test, and gene micro array analysis is as of yet not universally available.
Some symptoms and signs of gastric cancer were associated with a more grave prognosis. Dysphagia is a cardinal symptom for cancer of the gastric cardia. This location had worse prognosis than other locations of gastric cancers. Fatigue and weight loss are general symptoms of widespread disease for most types of malignant neoplasia. A palpable gastric tumour signifies a locally advanced tumour, and ascites is a sign of peritoneal metastases.
An improved survival rate has recently been reported in a randomized, controlled trial with neoadjuvant chemotherapy in operable gastric cancer Citation[23]. The figures indicate a 57% increase of 5-year survival, from 23 to 36%, and a possible effect of down staging in the treatment arm. It is likely that perioperative chemotherapy will be considered for routine use also in Norway. This implies that the treatment of operable gastric cancer must be done in close cooperation between surgical and oncological expertise and will also be limited to fewer hospitals.
A high hospital volume has been closely related to improved postoperative patient outcome in extensive surgical procedures like pancreatectomy and esphagectomy Citation[24]. For gastric cancer the best treatment results have also been reported in high volume hospitals with experienced surgeons, although good results can be obtained in low volume hospitals with experienced surgeons Citation[25]. The results of the present study were in line with what has been reported from larger centres in the Western world and indicate that good surgical craftsmanship may be done with experienced and dedicated surgeons in a medium sized hospital. In Norway, a redistribution of tasks between hospitals is taking place. At present, cardia cancer patients from this area are referred to the University Hospital of Trondheim for surgery, while gastric cancer from other locations is treated in Levanger. If the incidence of new operable cases of gastric cancer continues to decline, it is reasonable that these patients will be referred to even smaller number of centres in the future.
In conclusion, our results show that a medium sized hospital can achieve short and long-term outcomes that compares well with results from larger hospitals in the Western world.
The resection rate of 43% was low because so many were detected in advanced stages, and to detect more early stage cancers is a great challenge. Symtoms duration of longer than 6 months was associated with better long-term survival than short symptoms duration. Dysphagia, fatigue, weight loss, palpable tumour, ascites and anaemia at presentation were associated with a serious prognosis.
References
- Cancer in Norway 2004. http://www.kreftregisteret.no. 2006.
- Kunisaki C, Ishino J, Nakajina S, Motohashi H, Akiyama H, Nomura M, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13: 221–8
- Barchielli A, Amorosi A, Balzi D, Crocetti E, Nesi G. Long-term prognosis of gastric cancer in a European country: A population-based study in Florence (Italy). 10-year survival of cases diagnosed in 1985–1987. Eur J Cancer 2001; 37: 1674–80
- Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, et al. Survival after gastric adenocarcinoma resection: Eighteen-year experience at a single institution. J Gastrointest Surg 2005; 9: 718–25
- Damhuis RA, Meurs CJ, Dijkhuis CM, Stassen LP, Wiggers T. Hospital volume and postoperative mortality after resection for gastric cancer. Eur J Surg Oncol 2002; 28: 401–5
- ASA Physical Status Classification System. http://www.asahq.org/clinical/physicalstatus.htm. 2006.
- Hartgrink HH, van de Velde CHJ, Putter H, Bonenkamp JJ, Klein KE, Songun I, et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004; 22: 2069–77
- Arak A, Kull K. Factors influencing survival of patients after radical surgery for gastric cancer. A regional study of 406 patients over a 10-year period. Acta Oncol 1994; 33: 913–20
- Arak A, Lehtola J, Makela J, Tuominen H. Gastric cancer: Surgical management and prognosis. Ann Chir Gynaecol 1996; 85: 293–8
- Borch K, Jonsson B, Tarpila E, Franzen T, Berglund J, Kullman E, et al. Changing pattern of histological type, location, stage and outcome of surgical treatment of gastric carcinoma. Br J Surg 2000; 87: 618–26
- Degiuli M, Sasako M, Ponti A, Calvo F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 2004; 90: 1727–32
- Edwards P, Blackshaw GR, Lewis WG, Barry JD, Allison MC, Jones DR. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. Br J Cancer 2004; 90: 1888–92
- Hansson LE, Ekstrom AM, Bergstrom R, Nyren O. Surgery for stomach cancer in a defined Swedish population: Current practices and operative results. Swedish Gastric Cancer Study Group. Eur J Surg 2000; 166: 787–95
- Haugstvedt TK, Viste A, Eide GE, Soreide O. Norwegian multicentre study of survival and prognostic factors in patients undergoing curative resection for gastric carcinoma. The Norwegian Stomach Cancer Trial. Br J Surg 1993; 80: 475–8
- Jagoditsch M, Pertl A, Jatzko GR, Denk H, Stettner HM. Long-term outcome of stomach carcinoma achieved in an Austrian standard hospital with an oncologic focus. Chirurg 2001; 72: 822–31
- Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg 2005; 92: 1099–102
- Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric cancer surgery: Morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy–Japan Clinical Oncology Group study 9501. J Clin Oncol 2004; 22: 2767–73
- Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: Ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228: 449–61
- Viste A, Horn A, Hoem D, Øvrebø K. Results after surgical treatment of gastric cancer. Yearly meeting of the Norwegian Surgical Association. 2004.
- Tanaka K, Kiyohara Y, Kato I, Matsumoto T, Yamagata H, Kubo M, et al. Incidence and prognosis of gastric cancer in a population-based cohort survey: The Hisayama study. Scand J Gastroenterol 2004; 39: 459–63
- Stephens MR, Lewis WG, White S, Blackshaw GRJC, Edwards P, Barry JD, et al. Prognostic significance of alarm symptoms in patients with gastric cancer. Br J Surg 2005; 92: 840–6
- Maconi G, Kurihara H, Panizzo V, Russo A, Cristaldi M, Marrelli D, et al. Gastric cancer in young patients with no alarm symptoms: Focus on the delay in diagnosis, stage of neoplasm and survival. Scand J Gastroenterol 2003; 38: 1249–55
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. NEJM 2006; 355: 11–20
- Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–51
- Meyer HJ. The influence of case load and the extent of resection on the quality of treatment outcome in gastric cancer. Eur J Surg Oncol 2005; 31: 595–604