To the Editor
A 41-year-old man presented with lung mass revealed by chest radiograph in his medical check-up in October 2005. He was a healthy hepatitis B virus (HBV) carrier and was in good general condition without any respiratory symptoms. The patient reported that he smoked approximately 20 packs of cigarettes per year. Chest CT detected infiltrative mass encasing the right main bronchus, lobar bronchus and pulmonary artery with lymphangitic spread and enlargement of multiple lymph nodes. No notable abnormalities or metastatic bone lesions were seen on abdominopelvic CT-scan and whole body bone scan. The histopathologic diagnosis by fiberoptic bronchoscopy and lung biopsy was primary lung cancer; a poorly differentiated adenocarcinoma. After assessment, we concluded that this T4N2M0 stage IIIB lung cancer was unresectable (A, B). The patient received 30 mg/m2 vinorelbine IV on day 1 and 8, and 80 mg/m2 cisplatin on day 1 every 3 weeks.
Figure 1. Radiological images before cetuximab treatment. Chest CT before treatment (A, B) and after 1 cycle of chemotherapy (C, D), demonstrating aggravation of pericardial effusion, bilateral pleural effusion, and right lobe consolidation.
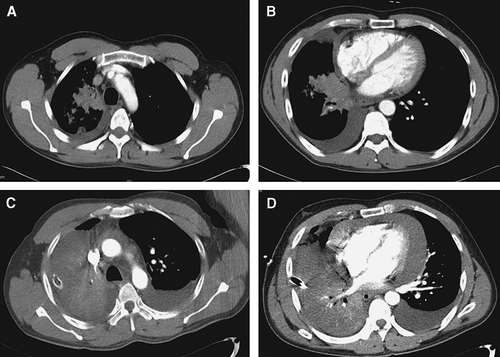
Two weeks after the initial vinorelbine and cisplatin chemotherapy, shortness of breath and abdominal discomfort were observed. Chest CT-scan showed bilateral pleural effusion and increased pericardial effusion with a maximum width of 3.6 cm. Pericardiocentesis was performed and malignant cells were present on pericardial fluid cytology.
In December 2005, chest CT-scan was repeated to evaluate the response of the initial chemotherapy. Bilateral pleural effusion was markedly increased and the right lung was totally collapsed. There was progression of the pericardial thickening (C, D). The overall response according to RECIST criteria was progressive disease. Immunohistochemical staining for EGFR demonstrated a positive reaction. Sequencing analysis showed that exon 19 of the EGFR gene contained a heterozygous in-frame deletion removing nucleotides 2 235 through 2 249.
We added the EGFR antibody cetuximab to the vinorelbine and cisplatin regimen. 400 mg/m2 cetuximab was loaded on the first day and doses of 250 mg/m2 were repeated weekly. After two cycles of chemotherapy with cetuximab the chest CT-scan showed improved malignant pleural and pericardial effusion and the treatment response was partial. The regimen of systemic chemotherapy with cetuximab was continued and was well tolerated except for grade 2 acne-like skin reaction.
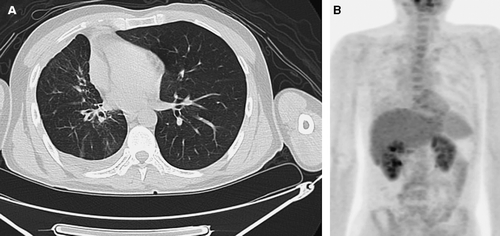
After eight cycles of treatment, fusion PET-CT scan was performed to reassess the response. There was no evidence of active viable tumor and pericardial effusion and only minimal FDG uptake at right upper and lower lung fields suggesting post-chemotherapy reaction (A, B). After nine cycles of systemic chemotherapy, radiotherapy was started in June 2006 for intensive local treatment in combination with weekly 250 mg/m2 cetuximab only. No evidence of progression has been seen during follow-up in the outpatient clinic, and treatment is continuing at the present time.
Figure 2. (A) Chest CT after 8 cycles of combination treatment with cetuximab demonstrating a dramatic reduction of pericardial effusion, pleural effusion and primary lung mass.(B) FDG PET scan showing no evidence of active viable tumor.
Virtually all NSCLC patients who show an initial response to first-line therapy eventually relapse. Unfortunately, patients with relapsed NSCLC may have poor prognosis and there are only limited treatment options. However, recent interest in the molecular biology of NSCLC has revealed the possibility of innovative biologic therapies that target different aspects of tumor development. In particular, most NSCLC cells express EGFR, which mediates the signaling pathway associated with increased cell proliferation, angiogenesis, and reduced apoptosis Citation[1]. EGFR-targeted therapy using monoclonal antibodies or tyrosine kinase inhibitors (TKIs) offer alternative treatment options for NSCLC.
Cetuximab (C225, Erbitux) is an immunoglobulin G1 mouse-human chimeric monoclonal antibody that inhibits the autophosphorylation of EGFR that causes cell cycle arrest. Cetuximab increases production of proapoptotic proteins and decreases levels of antiapoptotic proteins Citation[2]. It also elicits antibody-dependent cellular cytotoxicity Citation[3] and forms receptor-containing complexes that result in receptor internalization, an important mechanism attenuating receptor signaling. In this regard cetuximab is more powerful than the small molecular drugs that inhibit EGFR temporarily or reversibly Citation[4].
It has been demonstrated that these monoclonal antibodies markedly augment the anti-tumor effects of different classes of chemotherapeutic agents, including cisplatin, doxorubicin, paclitaxel, and topotecan Citation[5–7]. In contrast to the negative results of chemotherapy in combination with EGFR-TKIs in trial involving specific subsets of patients Citation[8], recent clinical trials of cetuximab and docetaxel in combination showed promising response rates in chemorefractory NSCLC patients Citation[9]. Phase II studies of cetuximab and concurrent chemotherapy for first-line therapy of EGFR-positive advanced stage NSCLC also showed positive response benefits over chemotherapy alone Citation[10].
To the authors’ knowledge, this is the first report of a dramatic response to cetuximab combined with a platinum-based regimen in an advanced NSCLC which was rapidly progressing during platinum-based chemotherapy. This treatment resulted in a rapid reduction of primary mass and pleural effusion after two cycles of combination therapy, and after eight cycles of treatment no viable tumor cells were detected in PET-CT scan. Moreover, except for an acne-like rash, this regimen did not exacerbate the side effects associated with chemotherapy. After completion of chemotherapy, we started radiotherapy with weekly cetuximab for intensive local treatment, based on several studies in which combinations of cetuximab with radiation produced synergistic growth inhibition in pre-clinical and clinical studies Citation[11]. Therefore, our case may clarify the role of cetuximab in patients with NSCLC followed by those of colorectal cancer and head and neck cancer.
In summary, we demonstrate that cetuximab can prevent rapid disease progression of NSCLC as chemotherapy has no time to work. It is hoped that further studies will elucidate mechanism of action of this agent and clinical role in overcoming chemoresistance in the management of patients with NSCLC.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R11-2000-082-03002-0).
References
- Yarden Y. The EGFR family and its ligands in human cancer signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001; 37(Suppl 4)S3–S8
- Kies MS, Harari PM. Cetuximab (Imclone/Merck/Bristol-Myers Squibb). Curr Opin Investig Drugs 2002; 3: 1092–100
- Naramura M, Gillies SD, Mendelsohn J, Reisfeld RA, Mueller BM. Therapeutic potential of chimeric and murine anti-(epidermal growth factor receptor) antibodies in a metastasis model for human melanoma. Cancer Immunol Immunother 1993; 37: 343–9
- Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem 1994; 269: 27595–602
- Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr, et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst 1993; 85: 1327–33
- Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res 1993; 53: 4637–42
- Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res 1999; 5: 909–16
- Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol 2004; 22: 777–84
- Kim ES, Mauer AM, Tran HT. A phase II study of cetuximab, an epidermal growth factor receptor (EGFR) blocking antibody, in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell cancer: Finla report [Abstract 2581]. Proc Am Soc Clin Oncol 2003; 22: 642
- Gatzemeier U, Rosell R, Ramlau R. Cetuximab (C225) in combination with cisplatin/vinorelbine vs. cispaltin/vinorelbine alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR) positive advanced non-small-cell lung cancer (NSCLC) [Abstract 2582]. Proc Am Soc Clin Oncol 2003; 22: 642
- Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 2001; 19: 3234–43