To the Editor
Bevacizumab (Avastin) is a recombinant, humanized anti-vascular endothelial growth factor (VEGF) monoclonal antibody that inhibits tumor angiogenesis. Randomized clinical trials have demonstrated significant improvement in survival for patients with advanced colorectal cancer, when treated with bevacizumab together with a 5-fluorouracil (5-FU) based combination chemotherapy Citation[1–3]. Bevacizumab treatment is associated with a unique side-effect profile, which includes hypertension, proteinuria, thromboembolic events, bleeding and impaired wound healing Citation[4]. In addition, bevacizumab treatment may be associated with severe intestinal complications, including perforations, which have been observed in up to 3% of the patients, colitis and diarrhea Citation[5–7]. We report here, for the first time, on high incidence of another severe intestinal complication, namely fistula formation. Moreover, we suggest an association between fistula formation and active pelvic disease and lack of response to bevacizumab.
The charts of all patients with metastatic or locally advanced rectal cancer, treated with bevacizumab at the Sheba Medical Center from the introduction of bevacizumab in January 2005 until this analysis in April 2006 were reviewed. Demographic and clinical data were obtained and stage was defined according to the 2002 American Joint Committee on Cancer Staging System for colon and rectal cancer Citation[8]. Information regarding therapy, including type of surgery, radiation therapy, chemotherapy and bevacizumab therapy were obtained from the patients’ charts and response to bevacizumab therapy and its duration were documented.
Between January 2005 and April 2006 13 rectal cancer patients were treated in our institution with bevacizumab together with 5-FU, leucovorin and irinotecan (FOLFIRI) regimen, for metastatic (12 patients) or locally advanced disease (one patient, ). Four of these patients developed either recto-cutaneous (three patients) or recto-vaginal fistula (one patient). Fistula formation occurred from 2 to 5 weeks following bevacizumab treatment. Three of the patients who developed fistula had prior operation and two of them received prior radiation therapy. Four of five patients with locally advanced disease suffered from fistula, compared to none of eight with distant metastases but without locally advanced disease. Progressive disease was also noted in all the patients who developed fistula but only in three of those who did not.
Table I. Demographic and clinical characteristics of rectal cancer patients with and without fistula formation.
During the same time period 53 colon cancer patients were treated by the same 5-FU, leucovorin and irinotecan (FOLFIRI) regimen. No fistula formation or perforation was noted among these patients.
Patient 1
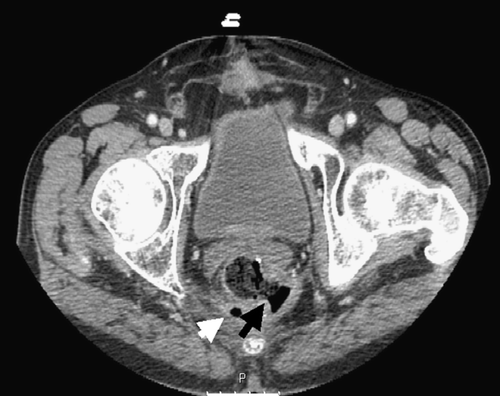
A 70-year-old man presented on July 2005 with rectal carcinoma. Clinical staging at diagnosis, as evaluated by transrectal ultrasonography (TRUS) was T3N0. The patient received neoadjuvant treatment consisting of radiation (45 Gy and boost of 5.4 Gy, fractions of 1.8 Gy) and 5-FU (500 mg/m2/d X5, on the first and last week of the radiation) and on September 2005 underwent a low anterior resection using the total mesorectal excision (TME) technique. Pathological analysis of the tumor revealed few microscopic residual foci of adenocarcinoma in the submucosa and the muscularis propria and involvement of all three identified lymph nodes; consistent with pathological staging T2N1. The postoperative course was unremarkable. Eight weeks after the operation multiple liver metastasis were identified and treatment with bevacizumab (5 mg/kg) and the FOLFIRI regimen every other week was started. Following the third course the patient experienced severe perianal pain and a large perianal abscess, containing large amount of pus and fecal content, was drained. A recto-cutaneous fistula was diagnosed () and the patient required a protective ileostomy. Radiographic evaluation revealed progressive liver disease and the treatment with bevacizumab and FOLFIRI was discontinued.
Figure 1. An abdominal computerized tomography of patient 1 was conducted after the third course of bevacizumab and FOLFIRI. The black arrow indicates a fistula originating from the left posterolateral wall of the rectum. Air bubbles in the perirectal area (white arrow) are suggestive of a perirectal abscess.
Patient 2
A 53-year-old woman presented on 2002 with rectal carcinoma. Low anterior resection of the rectum was conducted and the pathological staging was T3 with no tumor found in all eight identified lymph nodes. The patient was treated by adjuvant radiation therapy (45 Gy and boost of 5.4 Gy, fractions of 1.8 Gy) and chemotherapy (5-FU, 500 mg/m2/d X5, on the first and last week of the radiation), followed by chemotherapy alone for additional 4 months. In January 2005 the patient presented with liver and lung metastases and a pelvic mass. A CT guided biopsy of the pelvic mass confirmed the diagnosis of metastatic rectal carcinoma. Treatment with bevacizumab and FOLFIRI was started, but following a single course the patient noticed vaginal discharge and a recto-vaginal fistula was diagnosed. The patient underwent colostomy and 6 weeks after operation treatment with bevacizumab and FOLFIRI was continued. Evaluation after five courses, 2 months later, revealed progressive disease.
Patient 3
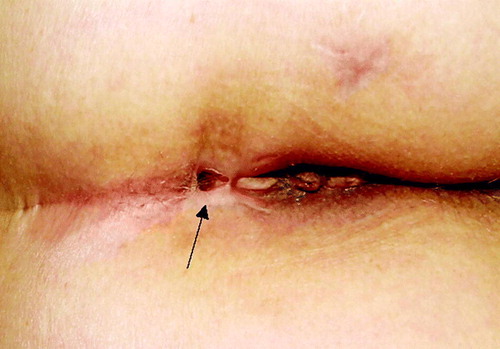
A 66-year-old woman was operated on September 2005 for rectal carcinoma and did not receive any form of adjuvant therapy. The patient immigrated to Israel and first presented to our institution on March 2006 with liver metastases and a pelvic mass. A biopsy of the mass was consistent with rectal adenocarcinoma and treatment was started with bevacizumab and FOLFIRI regimen every other week. Following two courses of therapy, the patient experienced severe pelvic pain and high fever, followed by the appearance of a recto-cutaneous fistula (). The patient received two additional courses of treatment but progressive disease was noted, both in the liver and pelvis and treatment was discontinued.
Patient 4
A 50-year-old man presented on March 2006 with rectal carcinoma and liver metastases. Treatment with bevacizumab and FOLFIRI regimen every other week was started. Following three courses of treatment the patient developed fever and CT-scan revealed a fistula, which penetrated the ischio-rectal adipose tissue and the levator-ani muscle. Enlargement of liver metastases was noted. Bevacizumab and FOLFIRI treatment were discontinued and antibiotic treatment was administered. The patient's condition improved and treatment with 5-FU, leucovorin and oxaliplatin (FOLFOX regimen) was started. We report here on four cases of fistula formation among metastatic rectal cancer patients while being treated with a bevacizumab-containing regimen. The time course between the start of bevacizumab treatment and the appearance of fistula was between 2 to 6 weeks and is similar to that observed recently for other severe bowel complications following bevacizumab treatment in rectal cancer patients Citation[5]. This short time course, especially when observed in two independent studies, is highly suggestive of a true association between bevacizumab treatment and severe bowel complications. However, due to the relatively small number of patients in our study, the possibility of an incidental finding cannot be ruled out.
The incidence of fistula formation among our patients was high. Recent studies also observed high incidence of complications in such patients. Lordick et al. reported three cases of severe complications among 22 patients (14%) with colorectal cancer Citation[5]. As all complications occurred only in rectal cancer patients, the incidence among rectal cancer patients is probably much higher. Willett et al. reported on severe complications in two of ten patients treated with bevacizumab. The complications were observed among five patients who received bevacizumab at a dose of 10 mg/kg, but not among the patients who received a dose of 5 mg/kg Citation[6]. The reported incidence of bowel perforation in recent large randomized studies was only up to 3% of patients Citation[4]. Only about 20% of the participants in these trials suffered from rectal carcinoma; and complications were not categorized by the primary disease, colon or rectal. Thus, the incidence of severe bevacizumab-induced bowel complications among rectal cancer patients cannot be determined from published data. None of the published studies reported on increased incidence of fistula. Perhaps fistula formation occurred but was attributed to the primary disease and not to bevacizumab treatment.
Three of our patients were operated for local disease and two of them received radiation therapy, as part of either adjuvant or neoadjuvant treatment. However, as radiation therapy is frequently administered as part of the treatment of early rectal cancer, it remains to be elucidated whether the occurrence of bevacizumab-induced complications is associated with prior radiation therapy or simply with local spread of the non-peritonialized rectum. Another possible contributing factor to bevacizumab toxicity may be irinotecan, which was administered to all our patients and is well known for its severe gastrointestinal side effects.
All our patients share two common features: active pelvic disease and lack of response to bevacizumab at any metastatic site, either hepatic or pelvic. How these two factors interact is currently unknown. We suggest that there is an active process of invasion and healing at the site of locally advanced disease involving bowel and bevacizumab prevents healing of tumor-induced damage to the bowel wall.
In other tumor types perforation of viscera or blood vessels has also been noted in patients treated with bevacizumab in the presence of active disease. A recent study of patients with advanced chemotherapy resistant ovarian cancer reported that 5 of 44 patients receiving single agent bevacizumab had gastro-intestinal perforation Citation[9]. In a study of lung cancer patients 6 of 67 patients treated with bevacizumab and carboplatin developed a major life-threatening bleeding event Citation[10]. These were associated with centrally located tumors which showed necrosis and cavitation.
While larger studies are needed in order to evaluate the association between active pelvic disease and lack of response to bevacizumab and fistula formation among rectal cancer patients, our data suggest that clinicians should be aware of the possibility of such an association, particularly in patients with local recurrence and previous radiation therapy.
References
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright J, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42
- Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–12
- Giantonio BJ, Catalano NJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer. Results from the Eastern Cooperative Oncology Group (ECOG) study E3200, Abstract. J Clin Oncol 2005; 23: S2
- Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology 2005; 69(Suppl 3)25–33
- Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 2006; 5: 553–66
- Lordick F, Geinitz H, Theisen J, Sendler A, Sarbia M. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: Report of three cases. Int J Radiat Oncol Biol Phys 2006; 64: 1295–8
- Willett CG, Boucher Y, Duda DG, di Tamoso E, Munn LL, Tong RT, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 2005; 23: 8136–9
- Colon and rectum In: American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. p 113–24.
- Cannistra SA, Matulonis U, Penson R, Wenham R, Armstrong D, Burger RA, et al. Bevacizumab in patients with advanced platinum-resistant ovarian cancer. J Clin Oncol 2006; 24: 5006
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22: 2184–91