Abstract
Recent studies have suggested that K-ras play an important role in the induction of COX-2 expression in tumor cells. In the present study, tumor samples of 89 gastric cancer patients were prepared in tissue microarrays and they were stained by immunohistochemistry with antibodies against COX-2 and K-ras. We investigated the relationship between the protein expressions of COX-2 and K-ras in gastric cancer and their significance as prognostic markers in gastric cancer patients. The over expression rate of COX-2 and K-ras in gastric cancer was 61.8% and 61.8% (55/89) of all the patients, respectively. There was a significant positive correlation between COX-2 and K-ras expression in gastric cancer. COX-2 and K-ras positivity were correlated with depth of invasion and lymph node metastasis, respectively. K-ras positivity was correlated with growth pattern. Patients with COX-2 and K-ras positive tumors had a poorer prognosis than those with COX-2 and K-ras negative tumors. Over expression of COX-2 and K-ras were closely correlated to prognostic of patients with gastric cancer and they educed synergistic effect with carcinogenesis and development in gastric cancer.
Cyclooxygenase-2 (COX-2) is known to be induced by fibroblasts, endothelial cells, monocytes, cytokines and some tumor types in response to growth factors Citation[1], and was speculated to be associated with the H-ras gene Citation[2]. The ras-transfected mammary epithelial cells show increased levels of both COX-2 mRNA and protein Citation[3]. Ras mutations are found in a wide variety of human malignancies and in ∼50% of colorectal carcinomas, and are associated with the progression of human malignancies Citation[4]. Such an association has also been suggested for COX-2 Citation[5], Citation[6]. Moreover, recent studies have suggested that COX-2 expression was strongly correlated with K-ras gene mutation in tumor cells Citation[7–10]. These findings prompted us to investigate the relationship between K-ras and COX-2 expression in human gastric cancer. In the present study, we investigated and analyzed COX-2 and K-ras expression, and the relationship between K-ras and COX-2 expression and prognosis of gastric cancer. The expression of K-ras and COX-2 was analyzed by tissue microarrays in the resected gastric cancer.
Materials and methods
Patients and tissue specimens
A total of 89 formalin-fixed paraffin-embedded primary gastric cancer specimens were obtained from Department of Pathology at the Lanzhou Medical College. The specimens were from patients who underwent gastrectomy for gastric cancer between 1987 and 2001. All of patients operated on had a D2 resection. The patients consisted of 68 men and 21 women, ranging in age from 22 to 70 years of age (median, 51years). None of the patients received chemotherapy or radiotherapy before the operation. All of the resected tumor specimens were formalin fixed, sectioned consecutively, and stained with hematoxylin and eosin for microscopic examination. Clinicopathologic data including sex, age, histologic type and degree of differentiation, depth of invasion, growth pattern and presence of regional lymph node metastasis. Lymph node metastasis was defined as positive when its number exceeded one. Survival data were collected retrospectively. The median follow-up period of the patients was 48 months (range, 1 – 224 months).
Tissue microarray construction and immunohistochemistry
For the construction of tissue microarrays, morphologically representative tumor areas regions for sampling were carefully selected and marked on the hematoxylin eosin slide. Thirty-six holes in a recipient block were created by using thin-walled stainless biopsy needle. The cylindrical core tissue samples 2.0 mm in diameter were retrieved from the selected region in the donor blocks and extruded directly into the recipient block with a solid stylet. The specimens were sampled with two core samples of the tumor. Sections of the resulting tumor tissue microarray block 4 µm thick were transferred to poly-L-lysine coated glass slides ().
For immunohistochemical study of COX-2 and K-ras expression in the tumor tissue, the sections were deparaffinized in xylene three times for 5 min each, descending alcohols for rehydration and then washed in distilled water. To enhance antigen retrieval, the sections were pretreated in a microwave oven for 5 min in 0.01 mol/l citrate buffer pH 6.0 and then cooled to room temperature. Thereafter, sections were blocked for endogenous peroxidase (SPkit, Maxin Biotech Inc) and incubated overnight at 4° C with primary antibodies(COX-2: rabbit polyclonal antibody, dilution 1:50, Santa Cruz; K-ras: rabbit polyclonal antibody, BAO282, Boster Biotech Inc). Then the sections were sequentially incubated with biotinylated secondary antibodies for 20 min at 37° C, and incubated with streptavidin–biotin-peroxidase for 20 min at 37° C. The peroxidase reaction was visualized by staining with 0.05% diaminobenzidine chromogen supplemented with 0.2% hydrogen peroxidase in PBS. Finally, the sections were counterstained with hematoxylin staining. Positive and negative controls were used for each set of experiments. Sections which had been confirmed to express COX-2 were used as positive controls. Negative control for every experiment was done by replacing the primary antibodies with PBS.
Immunohistochemical assessment
Tissue microarray slides were assessed under the light microscopy with the investigators unaware of the clinicopathologic variables. For COX-2 and K-ras, the percentage of target cells with cytoplasmic staining was recorded. Staining for COX-2 and K-ras was graded as described previously Citation[11]: grade 0 (negative), grade 1 (<5% tumor cells showed immunoreactivity), grade 2 (5 – 30% positive tumor cells) and grade 3 (>30% positive tumor cells). COX-2 and K-ras overexpression was defined as tumors with exceed grade 2 expression level. Necrotic areas were not taken into consideration.
Statistical analysis
Statistical analysis was performed with SPSS11.0 and a χ2-square test was used to analyze the relation between pathologic characteristics and immunohistochemical expression. Survivial curves were estimated by Kaplan-Meier method, and differences in survival were evaluated by log-rank test. The influence of each variable on survival was assessed by the Cox's proportional hazard model. Spearman was used to analyze correlation between COX-2 and K-ras expression in gastric cancers. A P value of less than 0.05 was accepted as significant.
Results
Expression of COX-2 and K-ras with relation to pathology
The expression of COX-2 and K-ras was primarily cytoplasmic in tumor cells of the gastric cancer (). The rate of COX-2 and K-ras expression were observed in 61.8% and 61.8% of 89 patients respectively. There was a significant positive correlation between COX-2 and K-ras expression (P=0.00)(). shows the relationship between pathologic parameters and the expression of COX-2 and K-ras.
Figure 2. (A) The expression of COX-2 protein in gastric cancer 400×, (B) The expression of K-ras in gastric cancer 200×
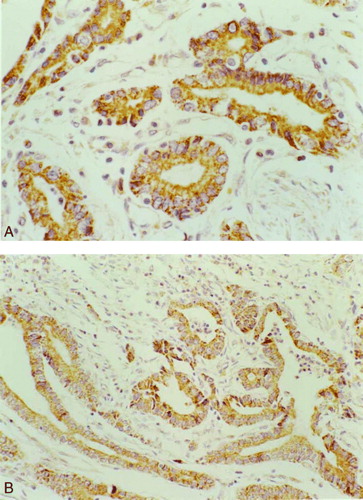
Table I. Relationship between COX-2 expression and K-ras expression
Table II. Correlation between clinicopathologic and expression of COX-2, K-ras
The relationship between COX-2, K-ras expression and the survival of patients with gastric cancer
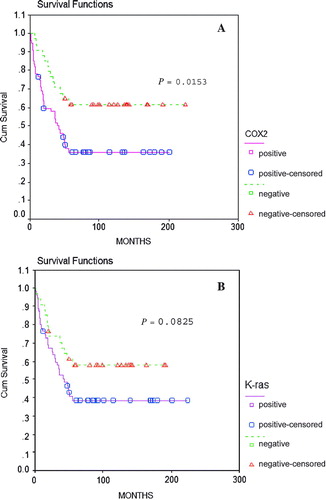
The prognosis of the 89 patients who underwent curative resection was studied. The Kaplan-Meier plots revealed that the prognosis of the patients with COX-2 positive tumors to be worse than that of those with negative tumors (P=0.0153), and the prognosis of the patients with K-ras positive tumors to be worse than that of those with negative tumors, but lacked in statistical significance (P=0.0825)().
Figure 3. Kaplan-Meier's survival curves. (A) The overall survival of gastric cancer patients with relation to COX-2 expression (log-rank test, P=0.0153) (B) The overall survival of gastric cancer patients with ralation to K-ras expression (log-rank test, P=0.0825)
We compared these data in univariate and multivariate settings. Univariate analysis indicated that depth of invasion, lymph node metastasis, sex and COX-2 expression were correlated to patients survival with gastric cancer. Multivariate Cox regression analysis for cancer-specific survival indicated that depth of invasion, lymph node metastasis, and sex were significant prognostic factors when all covariates were included in the analysis ( and ).
Table III. Univariate survival analysis of gastric cancers perfomed with Cox regression model
Table IV. Multivariate survival analysis of gastric cancers perfomed with Cox regression model
Discussion
Cyclooxygenase-2 is known to be induced by cytokines or growth factors Citation[1]. We were interested whether the COX-2 over expression in gastric cancer cells may be induced by specific genetic factors. COX-2 and K-ras gene are associated with the progression of human malignancies Citation[3], Citation[4], therefore, we investigated the relationship between K-ras and COX-2 expression in gastric cancer. Tissue microarray is an innovative tool in the research of prognostic markers, but there have been few reports on COX-2 and K-ras expression by tissue microarray.
In this study, tissue microarray was applied to evaluate the prognostic role of COX-2 and K-ras in patients with gastric cancer. The percentages of expression of COX-2 and K-ras were 61.8% and 61.8%, respectively, in the whole study population. These results were comparable with those of other studies of gastric cancer Citation[12–14]. Numerous studies indicate that cyclooxygenase activity and prostaglandin synthesis may be involved in promoting intestinal carcinogenesis. COX-2 expression may contribute to the synthesis of prostanoids, which have been related to carcinogenesis and tumor progression. The increased expression of COX-2 has been implicated in the development and progression of colorectal cancer Citation[9], Citation[15].
The depth of invasion and lymph node metastasis were reported to be the most important prognostic parameters in gastric carcinoma Citation[16]. The studies have found that over expression of COX-2 protein is associated significantly with lymph node metastasis Citation[17] and depth of invasion Citation[18]. Lee et al. Citation[19] have reported that COX-2 over expression was associated with tumor invasion beyond submucosa. In this study, COX-2 positive rate was significantly higher (P<0.05) in patients with serosa invasion than mucosa/submucosa invasion. Such results had also been observed for K-ras. Moreover, K-ras positive rate was significantly higher (P<0.01) in patients with infiltrating type growth than swell type growth. Significantly higher expression of K-ras was also observed in patients with lymph node metastasis than in those without (P<0.05). The results suggest that COX-2 and K-ras might enhance the progression and the metastatic potential of gastric cancer, and suppression of COX-2 and K-ras might be helpful in inhibiting tumor growth and invasion.
Although the precise role of COX-2 in Ras transformation is not understood completely, the induction of COX-2 by activation of Ras is well documented Citation[2], Citation[7–10], Citation[20]. We found that there was a significant positive correlation between COX-2 and K-ras expression in gastric cancer (P=0.00), thus suggesting that K-ras may induce COX-2 in the gastric cancer cells. Taylor et al. Citation[8] have shown that an activated K-ras oncogene leads to an up-regulation of COX-2 expression in human adenocarcinoma cells, suggesting that COX-2 is an important Ras target gene. From this point of view, and from the present study data, it seems likely that COX-2 expression is augmented by K-ras gene mutation. The K-ras gene may be a specific genetic factor for the induction of COX-2.
With regard to prognosis, Lee et al. Citation[19] found there was a trend favoring better survival in gastric cancers without COX-2 over expression. In our series, patients with COX-2 positive tumors had a poorer prognosis than those with COX-2 negative tumors (P=0.0153), and the prognosis of the patients and K-ras positive expression did not show statistical significance (P=0.0825). The results suggest that the presence of COX-2 expression, as well as conventional clinicopathologic factors, are prognostic indicators in patients with gastric carcinoma. Inhibition of COX-2 activity has an possible therapeutic benefit in the control of gastric carcinoma Citation[21], Citation[22]. Several studies have demonstrated that nonsteroical anti-inflammatory drugs (NSAIDs) or selective COX-2 inhibitors (for example SC-58125) could decrease cell growth in vitro and in vivo assays in cells expressing COX-2 Citation[6].
Sheehan et al. Citation[23] have reported that Dukes’ stage and number of metastatic lymph nodes remained as independent prognostic factors. We studied the effects of variables presumably associated with prognosis by multivariate analysis using the Cox model. As a result, the depth of invasion, lymph node metastasis and sex were independent prognostic factors. Sex may be interpreted as an independent prognostic factor of gastric carcinoma, however, it needs further study, for there are no similar findings reported.
To recapitulate, our results showed that the over expression of COX-2 was related to survival of the gastric cancer patients. COX-2 expression could not be an independent prognosticator. The significant positive correlation between COX-2 and K-ras expression in gastric cancer suggested that they play important synergism role in the oncogenesis and development of the gastric cancer.
References
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenase)-1 and –2. J Biol Chem 1996; 271: 33157–60
- Sheng GG, Shao J, Sheng H, Hooton EB, Isakson PC, Morrow JD, et al. A selective cyclooxygenase 2 inhibitor suppresses the growth of H-ras-transformed rat intestinal epithelial cells. Gastroenterology 1997; 113: 1999–03
- Subbaramaiah K, Telang N, Ramonetti JT, Araki R, Devito B, Weksler BB, et al. Transcription of cyclooxygenase-2 enhanced in transformed mammary epithelial cells. Cancer Res 1996; 56: 4424–9
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res 1989; 49: 4682–9
- Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett 2001; 172: 177–85
- Trifan OC, Hla T. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med 2003; 7: 207–22
- Sheng H, Shao J, Dubois RN. K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B. Cancer Res 2001; 61: 2670–5
- Taylor MT, Lawson KR, Ignatenko NA, Marek SE, Stringer DE, Skovan BA, et al. Sulindac sulfone inhibits K-ras-dependent cyclooxygenase-2 expression in human colon cancer cells. Cancer Res 2000; 60: 6607–10
- Liang JT, Huang KC, Jeng YM, Lee PH, Lai HS, Hsu HC. Microvessel density, cyclo-oxygenase 2 expression, K-ras mutation and p53 overexpression in colonic cancer. Br J Surg 2004; 91: 355–61
- Maciag A, Sithanandam G, Anderson LM. Mutant K-rasV12 increases COX-2, peroxides and DNA damage in lung cells. Carcinogenesis 2004; 25: 2231–7
- Sung JJY, Leung WK, Go MYY, To KF, Cheng ASL, Ng EKW, et al. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol 2000; 157: 729–35
- Uefuji K, IchiKura T, Mochizuki H. Expression of cyclooxygenase2 in human gastric adenomas and adenocarcinomas. J Surg Oncol 2001; 76: 26–30
- Kawabe A, Shimade Y, Uchida S, Maeda M, Yamasaki S, Kato M, et al. Expression of cyclooxygenase-2 in primary and remnant gastric carcinoma: comparing it with p53 accumulation, Helicobacter pylori infection, and vascular endothelial growth factor expression. J Surg Oncol 2002; 80: 79–88
- Van Rees BP, Saukkonen K, Ristimaki A, Polkowski W, Tytgat GNJ, Drillenburg P, et al. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol 2002; 196: 171–9
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997; 94: 3336–40
- Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma:well versus poorly differentiated type. Cancer 2000; 89: 1418–24
- Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. Would J Gastroenterol 2003; 9: 250–3
- Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tasunozaki H, et al. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer 2001; 91: 1876–81
- Lee TL, Leung WK, Lau JYW, Tong JHM, Ng KW, Chan FKL, et al. Inverse association between cyclooxygenase-2 overexpression and microsatellite instability in gastric cancer. Cancer Letters 2001; 168: 133–40
- Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, et al. Transforming growth factor-batal enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem 2000; 275: 6628–35
- Masferrer JL, Leahy KM, Koki AT, Zweitel BS, Settle SG, Woerner BM, et al. Antiangioginic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 2000; 60: 1306–11
- Qadri SSA, Wang JH, Redmond KC, O'Donnell AF, Aherne T, Redmond HP. The role of COX-2 inhibitors in lung cancer. Ann Thorac Surg 2002; 74: 1648–52
- Sheehan KM, Sheehan K, O'Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald D, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. J Am Med Assoc 1999; 282: 1254–7