Introduction
Squamous cell carcinoma of the head and neck, including the larynx, pharynx, and oral cavity is a relatively common human neoplasm. Increasing epidemiological studies indicate that these cancers are strongly linked to environmental factors such as chemical carcinogens present in tobacco and alcohol Citation[1], Citation[2]. The risk of head and neck cancer in smokers and alcohol users is more than twice that in non smokers and non alcohol users Citation[3], Citation[4]. Inter individual differences observed in the metabolism of carcinogens have been attributed to the genetic polymorphisms of genes encoding for enzymes involved in detoxification. This genetic variability seems to be associated with individual's susceptibility to some cancers, including head and neck cancer Citation[5–8].
N acetyl transferase 2 (NAT2) metabolizes aromatic amines, and is involved in the phase II metabolism of different compounds. Several mutations in the NAT2 gene such as G191A, T341C, G590A and G857A reduce the activity of the protein Citation[9], Citation[10], disturbing the metabolism of the corresponding substrates.
Glutathione S-transferase (GST) M1 and T1 are two enzymes of GST family that catalyze the reduction of several electrophilic substrates, facilitating the excretion of these compounds. Both of them are polymorphic. The GSTM1*0 (GSTM1 deficiency) and GSTT1*0 (GSTT1 deficiency) allele represent a deletion of the GSTM1 and GSTT1 gene and result in a loss of enzymatic activity Citation[11], Citation[12].
The aim of this study was to investigate the influence of deletion polymorphisms at GSTM1, GSTT1 genes and substitution polymorphisms at NAT2 gene (T341C and G590A), on susceptibility to head and neck cancer in a Tunisian population.
Patients and methods
Study groups
We performed a case control study: The case group consisted of 64 newly diagnosed patients with confirmed primary squamous cell carcinoma of head and neck at histology (nasopharyngeal (n = 46), laryngeal (n = 11), lips (n = 3), tongue (n = 2) and oropharyngeal (n = 2)). All patients were recruited at Cancer Institute of Tunis. This group included 42 men and 22 women (mean age: 50.7±12.27 years; 17–80 years). The healthy group consisted of 160 individuals (72 men and 88 women) without any prior diagnosis of cancer, recruited among the professional surrounding (mean age: 53.6±8.89 years; 40–70 years).
Methods
Genotyping
DNA was isolated from leukocytes in 10 ml of peripheral blood. The presence of the GSTM1 null and GSTT1 null deletion was screened by using a multiplex PCR procedure using primers described in . The amplification of albumin gene provides a positive control for each reaction. The 50 µl reaction mixture contained: 250 ng of genomic DNA, 22 pM for each primer, 200 µM of each dNTP, 2U of Taq DNA polymerase. Cycling conditions consisted of 5′at 94°C for initial denaturation and 2′at 64°C for initial hybridization followed by 30 cycles of extension for 30′′at 72°C, denaturating for 20′′at 94°C, hybridization for 20′′at 64°C and final extension at 72°C for 7′. Ten microliter of each PCR product was analyzed for efficient amplification on an agarose gel at 1%.
Table I. Primer sequences used for PCR
A PCR-RFLP method was used to detect substitution polymorphisms at NAT2 gene. Two fragments of 835 bp and 243 bp of NAT2 were amplified with a PCR procedure previously described Citation[13]. The G590A mutation was detected by the loss of TaqI restriction site in the 835 bp PCR product. The T341C substitution was detected by AccI cleavage of the 243 bp product.
Statistical analysis
The χ2 test was used to assess the distribution of allele frequencies between groups. Odds ratio (OR) and 95% confidence intervals (CI) were calculated using epi_info 6 logiciel to estimate relative risk association with some genotypes.
Results
Frequencies of homozygous deletion of GSTM1 and GSTT1 genes did not differ significantly from patients and controls () ().
Figure 1. PCR multiplex for genotyping GSTM1 and GSTT1. Lane 1: GSTM1 null and GSTT1 null; lane 2: GSTM1 null; lane 3: GSTT1 null; lane 4: corresponds to an individual who is positive for both GSTM1 and GSTT1
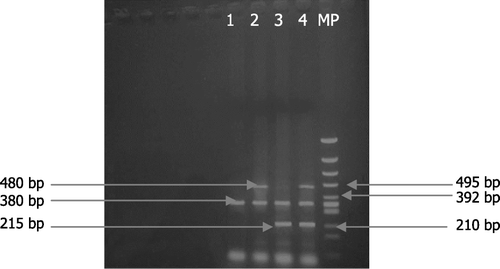
Table II. Frequencies of GSTT1 and GSTM1 null genotypes in patients and controls
Frequencies of G590A and T341C mutations in NAT2 gene are listed in .
Table III. Frequencies of G590A and T341C mutations in patients and controls
A significant difference was found between patients and controls for T341C mutation (p < 0.05) (). Calculated OR showed an association between T341C mutation and increased risk of head and neck cancer.
When the patients were stratified by disease site, the T341C mutation's frequency for the patients with squamous cell carcinoma of nasopharynx, larynx, lip, tongue or oropharynx were not statistically different from that of total patient population ().
Table IV. Frequency distribution of T341C mutation by disease site.
Discussion
GST and NAT2 are important phase II detoxifying enzymes. Total or partial deficit of these enzymes lead to a reduced capacity of detoxifying chemical compounds whose accumulation may promote cancer development.
We found no association between GSTM1 and GSTT1 null genotypes and head and neck cancer risk. Several studies related to association between GST genotypes and head and neck cancer are rather conflicting. Some reported that polymorphic variants of the GSTM1 and GSTT1 genes result in no significant contribution to head and neck cancer development Citation[3], Citation[14–17]. While others suggested that GSTM1 and/ or GSTT1 genes deletion might be risk factors for developing head and neck carcinoma Citation[6], Citation[18]. Discrepancy between these studies may be ascribable to ethnic difference in allelic frequencies of the GSTM1 and GSTT1 polymorphism or to factors such as diet, alcohol consumption, smoking habit Citation[19]. It is well known that variation in the geographic and ethnic distribution between cases and control individuals among studies may be a considerable bias, which might confound the results of pooling analysis Citation[20], Citation[21]. Z ye et al. Citation[22] have observed such an imbalance in geographic and ethnic distribution. For GSTM1 status, the risk of head and neck cancer is higher in African-American and Asians than in whites, while the risk of head and neck cancer is higher in Asia than in America and Europe. Similarly, for GSTT1 status, the risk of head and neck cancer is higher in America than in Europe and Asia. However, the risk of head and neck cancer seems consistent in the different ethnic group. Thus, the large differences in prevalence of enzyme's detoxification's polymorphisms in different ethnic group may influence the susceptibility to cancer by modification of enzyme's activity on environmental carcinogens.
We also analyzed the link between NAT2 polymorphisms and susceptibility of head and neck cancer. T341C mutation emerged as a significant risk factor for such cancer. Our findings are in accordance to the reported association between squamous cell carcinoma and T341C mutation Citation[23]. This is probably due to the great reduction in acetyltransferase 2 catalytic activity in relation with the T341C mutation in NAT2 gene Citation[24].
Conclusion
Inter individual differences observed in the metabolism of carcinogens have been attributed to the genetic polymorphism of genes which encode for enzymes involved in detoxification. This genetic variability seems to be associated with the individual's susceptibility to head and neck cancer. Our results showed no association between GSTM1 and GSTT1 null genotypes and susceptibility to head and neck carcinoma. T341C mutation of NAT2 gene was found to be associated with an elevated risk for head and neck cancer in Tunisian population.
References
- Cloos J, Spitz MR, Schaniz SP, et al. Genetic susceptibility to head and neck squamous cell carcinoma. J Nail Cancer Inst. 1996; 88: 530–5
- Risch A, Ramroth H, Raedts V, et al. Laryngeal cancer risk in Caucasians is associated with alcohol and tobacco consumption but not modified by genetic polymorphisms in class I alcohol dehydrogenases ADIIIB and ADIIIC, and glutathione S-transferases GSTM1 and GSTT1. Pharmacogenetics 2003; 13: 225–30
- Olshan AF, Weissler MC, Walson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYPIA1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000; 9: 185–91
- Kietthubthew S, Sriplung H, Au WW. Genetic and environnemental interactions on oral cancer in southern Thailand. Environ Mol Mutagen. 2003; 37: 111–6
- Chen C, Ricks S, Doody DR, Fitzgibbons ED, Porter PL, Schwartz SM. N-Acetyltransferase 2 polymorphisms, cigarette smoking and alcohol consumption, and oral squamous cell cancer risk. Carcinogenesis. 2001; 22: 1993–1999
- Nizar- VS, Vaughan TL, Burt RD, Chen C, Berwik MG, Swanson M. Glutathione S transferase M1 and susceptibility to nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 1999; 8: 547–551
- Murat Aoenal M, Laiafer Tamer PhD, , Nurcan Aras Ates PhDet al Glutathione S-transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinoma. Am J Otolaryngol 2004;25:318–22.
- Shama C. Buch, Perin N. Notani, Rajani A. Bhisey. Polymorphism at GSTM1, GSTM3, and GSTT1 gene loci and susceptibility to oral cancer in an Indian population. Carcinogenesis. 2002;23:803–807.
- Butcher NJ, Boukouvala S, Sim E, Minchin RF. Pharmacogenetics of the arylamine N-acetyltransferases. The pharma J 2002; 2: 30–42
- Hamdy SI, Hiratsuka M, Narahara K, et al. Genotype and allele frequencies of TPMT, NAT2, GST, SULTIAI and MDR-I in the Egyptian population. Br J Clin Pharmacol 2003; 55: 560–569
- Seidegard J, Pero R. the hereditary transmission of the high GST activity towords trans-stilbene oxide in the human mononuclear leukocytes. Human genetics 1985; 69: 66–68
- Pemble S, Schroeder K, Spencer S, et al. Human glutatione s-transferase theta (GSTT1): cDNA cloning and the charachterization of a genetic polymorphism. Biochem J 1994; 300: 271–6
- Longuemaux S, Deloménie C, Gallou C, Méjean A, Dupret JM. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res 1999; 59: 2903–2908
- Hahn M, Hagedorn G, Kuhlisch E, Schackert KH, Eckelt U. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer. Oral Oncology 2002; 38: 486–490
- Mc Williams JE, Evans AJ, Beer TM, et al. Genetic polymorphisms in head and neck cancer risk. Head Neck 2000; 22(6)609–17
- Park LY, Muscat JE, Kaur T, et al. Comparison of GSTM polymorphisms and risk for oral cancer between African-Americans and caucasiens. Pharmacogenetics 2000; 10(2)123–31
- Hahn M, Hagedorn G, Kuhlisch E, Schackert KH, Eckelt U. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer. Oral Oncology 2002; 38: 486–490
- Gronau S, Koning Creger D, Rettinger G, Riechelmann H. GSTM1 gene polymorphism in patients with head and neck tumors. Laryngorhinootologie 2000; 79(6)341–4
- Miki S, Takayuki S, Toshiyuki I, Teruo A. Genetic polymorphism of drug-metabolizing enzymes and susceptibility to oral cancer. Carcinogenesis 1999; 20: 1927–1931
- Garte S. the role of ethnicity in cancer susceptibility gene polymorphisms the example of CYP1A1. Carcinogenesis 1998; 19: 1329–32
- Mareux PM, Vineis P, Rothman N. NAT2 slow acetylation and bladder cancer risk, meta-analysis of 22 case-control studies concludedin the general population. Pharmacogenetics 2000; 10: 115–22
- Ye Z, Song H, Guo Y. Gluthathione S-transferase M1, T1 status and the risk of head and neck cancer: a meta-analysis. BMJ 2004; 41: 360–365
- Gonzalez MV, Alvarez V, Pello MF, Menéndez MJ, Suarez C, Coto E. Genetic polymorphism of N-acetyltransferase-2, gluthatione S-transferase-M1, and cytochromes P450IIE1 and P450IID6 in the susceptibility to head and neck cancer. J Clin Pathol 1998; 51: 294–298
- Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutation Res 2002; 506–507: 65–77