Abstract
Trastuzumab has shown activity in early breast cancer patients that overexpress HER2. Significant resources have to be allocated to finance this therapy, underlining the need for cost-effectiveness analysis. A model was set up, societal costs were calculated and the discount rate was 3%. Life expectancy data were based on the literature and prolonged according to qualified guess (10% and 20% absolute improvement in overall survival (OS)). The comparator was the FEC100 regimen. The median additional health care cost per patient treated was €33 597. The yielding cost per life year gained (LYG) was €15 341 with a 20% improved OS and €35 947 with 10% improved OS. The corresponding net health care cost per quality adjusted life year (QALY) was €19 176 and €44 934. Including all resource use the figures were €8148 and €30 290 per LYG. Sensitivity analyses documented survival gain, price of trastuzumab, production gain and discount rate to be the major factors influencing cost-effectiveness ratio. Trastuzumab is indicated cost effective in Norway.
Breast cancer is the most common cancer among women in North America and throughout Europe. The high incidence makes the treatment of breast cancer a significant financial burden to the health care systems worldwide Citation[1]. HER-2/neu amplification is recognized in 20–30% of breast cancers Citation[2–4] and is associated with an aggressive form of the disease with significant shortened disease-free survival and overall survival Citation[5], Citation[6]. It is also an important factor predicting response to certain chemotherapy regimens and hormonal treatment Citation[7]. The trans-membrane receptor has been a goal for targeted manipulation with humanized murine monoclonal antibody (MAb). The most known antibody in today's clinical practice is the humanized murine antibody trastuzumab (Herceptin®: Genentech, Inc, So. San Fransisco, California). Currently other compounds are in the pipeline.
So far, drug costs in cancer therapy have constituted a minor share of the total resources spent on health care. Yet, the introduction of a panel of MAbs for different cancer forms may change the scenario. In Norway, the drug costs at oncology units have increased by about 30% annually for the last 3 years, mainly due to the implementation of MAbs.
While trastuzumab monotherapy Citation[8], Citation[9] had moderate effects in advanced breast cancer, trastuzumab improved overall time to progression but also survival when administered in concert with chemotherapy Citation[10], Citation[11]. Recently, four trials reported trastuzumab added to chemotherapy to reduce short-term relapse rate by approximately 50% compared to chemotherapy alone Citation[12–14]. While these results suggest potential long-term survival benefits, the high costs of this therapy call for studies enabling cost-effectiveness or cost-utility. As follow-up time is short, model based health economic analysis may be a supporting tool for administrators, authorities and insurers that have to decide whether to cover the significant amounts or not. This study explores cost-effectiveness of including trastuzumab in adjuvant breast cancer treatment in Norway.
Material and methods
The model is depicted in . A marginal analysis was employed, comparing the incremental costs of this new treatment as compared with – or in addition to – current standard treatment as proposed by Drummond Citation[15]. The total cost includes incremental health care cost (C1=drug cost, administration costs, cost of HER-2 analysis, cost of hospitalisation, cost of out-patient therapy), patient/family costs (C2=travelling) and costs in other sectors (C3=production losses). While travelling costs are covered by the health care system in Norway, it is reported here as patient/family costs in accordance to standard practice in this literature. The economic benefits will be in terms of health care savings (S1=avoided resources spent on treatment of relapses in the trastuzumab containing arm) and in terms of savings in other sectors (S2=production gains). The effectiveness (E) can be calculated as the difference in life years gained (LYG) employing the prior regimen (E1) (fluorouracil, epirubicin, cyclophosphamide – FEC100) and the new standard therapy (E2) (FEC100→trastuzumab).
Figure 1. The figure shows the model. Only the cost of the prevented relapses is counted in the analysis. Adjuvant trastuzumab is calculated saving 10 or 20% from death of metastatic breast cancer (death 0.8 or 0.9). 1st line therapy (paclitaxel/trastuzumab or docetaxel/trastuzumab) in metastatic setting was calculated administered in the time span 1–6 years following adjuvant chemotherapy (ACT) and 90% (0.9) were concluded candidates for 1st line therapy. 2nd line therapy (vinorelbine or vinorelbine/trastuzumab) was calculated administered in the time span 2–8 years following ACT and two-thirds (0.67) of patients undergoing 1st line therapy was candidates for 2nd line therapy. 3rd line therapy (capecitabine) was calculated administered 4–10 years following ACT and two-thirds (0.67) of patients undergoing 2nd line therapy was candidates for 3rd line therapy. . The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to trastuzumab.
![Figure 1. The figure shows the model. Only the cost of the prevented relapses is counted in the analysis. Adjuvant trastuzumab is calculated saving 10 or 20% from death of metastatic breast cancer (death 0.8 or 0.9). 1st line therapy (paclitaxel/trastuzumab or docetaxel/trastuzumab) in metastatic setting was calculated administered in the time span 1–6 years following adjuvant chemotherapy (ACT) and 90% (0.9) were concluded candidates for 1st line therapy. 2nd line therapy (vinorelbine or vinorelbine/trastuzumab) was calculated administered in the time span 2–8 years following ACT and two-thirds (0.67) of patients undergoing 1st line therapy was candidates for 2nd line therapy. 3rd line therapy (capecitabine) was calculated administered 4–10 years following ACT and two-thirds (0.67) of patients undergoing 2nd line therapy was candidates for 3rd line therapy. Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to trastuzumab.](/cms/asset/823083b7-7b76-40fd-a39a-6f1304a94ccc/ionc_a_209627_f0001_b.gif)
Various assumptions can be made in a cost-effectiveness analysis (CEA) depending on which costs and economic consequences that are accounted for. A narrow analysis accounts for health care costs and consequences only, i.e. C1–S1. A broad analyses include indirect costs and production gains (PG) as well, something that provides an estimation of net societal costs; i.e. C1+C2+C3−S1−S2. In a CEA, costs are compared with the incremental health gains; E2−E1 – as measured in LYG or quality adjusted life years (QALY). The exact resource consequence of using trastuzumab in the adjuvant setting is currently unknown. However, modelling can offer support to decision makers as long as it reflects real-world conditions Citation[16].
Treatment and comparator
Trastuzumab is additive in its current use and do not replace any existing therapy. Thus, we compare FEC100 [fluorouracil, epirubicin (100 mg/m2), cyclophosphamide] regimen administered for six cycles on a 3-weekly basis Citation[17] to the same regimen followed by trastuzumab 3-weekly administration for 17 cycles. This practice is according to the Norwegian Breast Cancer Group (NBCG) guideline as in November 2005 (www.nbcg.net). The NBCG has estimated approximately 12% of the Norwegian patients being candidates for adjuvant trastuzumab.
Trastuzumab is administered at a loading dose of 8 mg/kg following adjuvant chemotherapy. It is continued with a 3-weekly (6 mg/kg/3w) interval for one year (16 courses). Trastuzumab therapy is initiated within 7 weeks following end of adjuvant chemotherapy or within 6 weeks after finishing radiotherapy Citation[13].
Effectiveness
While there are several ongoing studies Citation[18] in this setting, so far the results from four trials Citation[12–14], Citation[19] have been reported in peer-reviewed journals. This include the international HERA trial Citation[13] and the combined analysis of the two North American studies (National Surgical Adjuvant Breast and Bowl Project-NSABP B31 and North Central Cancer Treatment Group NCCTG N9831) Citation[12]. One study has so far not been published in a peer-reviewed journal. The study by Joensuu et al. Citation[14] includes a limited number of patients and is the only one with a short time trastuzumab regimen. Due to this fact, we did not run any estimates based on the study.
So far, only one study Citation[12] has documented an improved overall survival (hazard ratio, 0.67; 95% confidence interval, 0.48 to 0.93; p = 0.0015). Based on all reports and employing distant relapse-free survival (DRFS) as a surrogate of future overall survival rates, we decided to employ a 10% and a 20% absolute improvement level in overall survival caused by trastuzumab (). The improved survival figure was in addition to a stated level and assumed to be reached at 10 years follow-up. Furthermore, the benefit was equally distributed over the whole ten-year period. To reflect the non-trastuzumab arm, the 30 years’ follow-up data of adjuvant therapy reported by Bonadonna and coworkers Citation[20] were plotted. While stage migration due to better classification of axillary status may move patients from the node negative toward the node positive group improving the prognosis for both due to a “Will Rogers” effect, HER2 positive tumors have a very poor prognosis, thus anticipated the poor prognosis related to this subtype to compensate for migration in N+ status Citation[5], Citation[6], Citation[21]. Bonadonna et al. Citation[20] disclosed at 25 years follow-up after adjuvant CMF an overall survival of 40%. Since then, anthracycline containing regimens have further improved the survival figures by 3–7% Citation[17]. A “stop and drop” situation is employed at 20 years follow-up. Life years gained can then be estimated by calculating the area between the FEC curve and the 10% and 20% improvement in absolute 10 years overall survival (). In a 20 years perspective 1.5 (10%-alternative) and 3 (20%-alternative) undiscounted life years are gained depending on alternative chosen. The four-year follow-up results from the joint study Citation[12] are added to illustrate the present short time knowledge in this setting.
Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to tratuzumab.
![Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to tratuzumab.](/cms/asset/e3da0350-513f-4ee1-bdcc-70c03a3a6121/ionc_a_209627_f0002_b.gif)
The median age of women undergoing adjuvant chemotherapy in Norway is 50 years Citation[22]. This is also in accordance with the 49 years reported in the HERA study Citation[13]. The number of primary breast cancer patients in Norway is shown in ten-year cohorts in . According to the recommendations for adjuvant chemotherapy (www.nbcg.net), fewer patients aged above 55 years of age will undergo adjuvant chemotherapy. Including only the ER- and ER (poor) patients, the “weight” in terms of the number of candidates for adjuvant chemotherapy in this group is reduced by 50%. In our model, this was compensated for by reducing the cohort aged 50–60 years by 25% and the cohort aged 60–70 years by 50%. Based on this information, life expectancy following adjuvant trastuzumab therapy is 26.53 years (). This figure is based on the general Norwegian population. Due to potential increased death rate related to long-term side effects (heart disease and secondary malignancy) we reduced expected survival benefit to an average of 20 years. This assumption is also supported by a prior study in this field Citation[17].
Table I. Effectiveness: The table illustrates the calculation of life expectancy following adjuvant chemotherapy containing trastuzumab in early breast cancer.
Costs
All costs are calculated according to Norwegian unit costs and converted to Euros at the rate of 1€ = 8.02 NOK as of February 6th 2006 (www.norges-bank.no).
Costs may be divided into health care costs, costs related to patient/family and costs in other sectors Citation[15]. The health care costs in adjuvant breast cancer therapy are mainly related to hospitalisation, out-patient treatment and drugs. Costs related to patient/family are mainly due to travelling. The important costs in other sectors are production losses.
The breast-cancer model () is set up from diagnosis to time to death. This includes the following phases: Diagnosis of breast cancer, surgical treatment (breast conserving surgery/mastectomy), adjuvant chemotherapy, radiotherapy, hormonal therapy and treatments in the metastatic phase. In an incremental CEA, only costs differing between the two groups need to be included. Adjuvant trastuzumab therapy have no influence on diagnosis, surgery, radiotherapy and chemotherapy in the primary setting (as it is added in a later phase) and the early phases were thus calculated zero in both arms. Details are shown in .
Table II. The costs per patient treated (in €). Only the costs and savings differing between the two adjuvant regimens (standard FEC100 and FEC100 + Trastuzumab) are included.
Most authors employ discount rates between 3% and 5% to incorporate future health outcomes and costs Citation[23]. While 5% has been standard practice, most contemporary studies have applied 3%.We used a 3% discount rate and included 0% and 5% in the sensitivity analysis.
Health care costs (C1)
a) Trastuzumab costs
The doses of trastuzumab employed in this model are described above (treatment and comparator) Trastuzumab is delivered in 150 mg standard units at a cost of €822/unit. Thus in women having a weight of more than 75 kg, 4 units (5 units for the first cycle) are necessary for a sufficient dose. To indicate the number of units needed, we registered the weight of all women with early breast cancer referred to the out-patient unit at the University Hospital of North Norway (UNN) in January and February 2004 for adjuvant chemotherapy. Twenty-three percent had a weight of more than 75 kg (mean 70.2 kg, range 56.5–92 kg). This weight is in accordance with the mean weight among Norwegian adult women of 69.5 kg according to Statistics Norway (www.ssb.no). Current practice indicates that most clinicians do not increase the number of units on economic reasons, even for women with a body weight slightly above 75 kg. Furthermore, patients may be clustered and trastuzumab left over when one patient's dose is prepared may be implemented in another's infusion. We therefore employed a qualified guess by two experienced medical oncologists at the Department of Oncology, UNN assuming 15% of the patients need an additional drug unit Citation[1]. Data from the HERA study has shown an 8.5% treatment withdrawal (www.asco.org). The reason was safety in 6% and refusal in 2.5% of the participating women. Based on these figures, we included a dose intensity of 94% in our model. As all costs occur within the first year, they were not discounted. Based on all information, the mean drug cost per patient treated with trastuzumab was calculated €42 354. Details are shown in Appendix 1. This figure together with the additional cost items discussed below is listed in .
b) Assessment of HER-2 status
There are two major alternatives for the assessment of HER-2 status, immunohistochemical (IHC) assay (i.e. HerCep test – DAKO, Carpinteria, CA) or in situ hybridization using fluorescence (FISH) or chromogen (CISH) detection. Whereas IHC is inexpensive, available and easily performed in clinical pathology labs, FISH/CISH is more predictive and therefore employed to confirm IHC results at the 2+ level. Whereas the cost of analysis for HER-2 status employing IHC is €30.9 /test, this test is applied to all breast cancers on a standard basis, as the result may influence choice of adjuvant endocrine treatment (www.nbcg.net) and the epirubicin dose of the FEC regimen (FEC100 vs FEC60). The cost of IHC, FISH/CISH was therefore excluded from the analysis.
c) Cardiac check-ups
By adding trastuzumab to standard chemotherapy in the adjuvant setting, new side effects and an altered need for hospitalisation occur. Congestive heart failure is the most important factor in this setting. According to national guidelines, MUGA scan should be performed every 12th week during adjuvant trastuzumab therapy. The cost of these five check ups is €330.
d) Administration costs at the pharmacy
For every drug prepared at the pharmacy for intravenous therapy, an administration cost is charged (€20.4). For a median number of 16 cycles (17*0.94), the pharmacy cost adds to €326.
e) Out-patient clinic costs
The introduction of adjuvant trastuzumab alters the number of visits to the out-patient clinic. According to recommendations, the dose of trastuzumab is administered as an i.v. infusion for 90 min. To reflect this cost, we added the tariff of the NHA Citation[24] for prolonged treatment to the standard tariff of out-patient visits. The total cost for 17 visits to the out-patient clinic employing 94% dose intensity was €2192 (Appendix 1). Patients not undergoing adjuvant trastuzumab are offered two follow-ups the first year. This cost of €98 is included in .
f) Treatment of congestive heart failure
Adding trastuzumab to standard chemotherapy in the adjuvant setting introduces new side effects. Congestive heart failure is the most important factor in this setting and trastuzumab seems to be most cardiotoxic when given concomitant with doxorubicin Citation[10], Citation[12], Citation[22], Citation[23]. However, when the drug is administered sequentially to chemotherapy, the risk seems to be in the 0.5–2.2% range (HERA/N9831). In the report from the HERA study, 7.1% had a decrease in left ventricular ejection fraction (LVEF) by 10 EF points and LVEF below 50%. Cardiologists documented congestive heart failure in 0.5% of the patients. The Diagnosis Related Groups (DRG) 127 Citation[25] was employed to reflect this cost of €23.
Travelling (C2)
According to Norwegian guidelines, each hospital has to cover patient transportation costs. Many patients are transported by taxi and others go by public transportation or use their own car. Based on a study by Norum and Holtmon Citation[17] and the national tariff, average transportation costs related to one year of adjuvant trastuzumab were estimated to €1249. The corresponding cost of two regular visits for patients not undergoing adjuvant trastuzumab therapy was €157.
Costs in other sectors (C3)
The indirect costs in this setting are production losses. The median age of women receiving trastuzumab was 49 years in the HERA Citation[13] as well as in the two American trials Citation[12]. Women undergoing chemotherapy for early breast cancer are temporarily out of work receiving financial compensation from the National Insurance Administration (NIA). According to Statistics Norway (www.ssb.no), 82.8% of women aged 50–54 years are in the workforce. The employer's annual labour costs (including income, pension and social costs) were used as a measure of the production value. The 2004 mean figure was €41 958/year per woman in the work force (Statistics Norway). The production losses during 17 visits were then calculated at €2272. The total raised cost due to adjuvant trastuzumab was calculated €48 491 ().
Economic consequences – savings
Improved outcome for early cancer treatment may reduce costs of therapy in the metastatic setting. At present there are no national databases that can show the treatment cost of metastatic breast cancer in Norway. If available these data would have been based on treatment practice more than a decade ago (prior to the introduction of trastuzumab as well as novel expensive cytotoxic compounds) and thus no longer valid. We therefore had to estimate this cost. Based on today's knowledge we used a survival gain estimate of either 10% or 20% in our model. Details are shown in . Due to performance status, cardiac status, age and women's preferences, some women will not be candidates for trastuzumab therapy in the metastatic setting. We assumed 90% of HER2 positive patients not exposed to trastuzumab in the adjuvant setting to be treated with a trastuzumab-paclitaxel regimen in the first line metastatic setting, and the doses were calculated according to a median body surface area of 1.75 m2Citation[17]. The practice at the UNN has mainly been paclitaxel (80–90 mg/m2) and trastuzumab (4 mg/kg 1st dose and then 2 mg/kg) on a weekly basis Citation[1]. In a prior study Citation[10], the median time in study was 40 weeks and the median number of doses of trastuzumab was 36 (range 1–98). The savings related to trastuzumab (20% improved overall survival (OS), 90% dose intensity (DI)) was calculated to €5764. The figure for paclitaxel (price list February 2006) was €2411. The out-patient clinic cost was €884 and the pharmacy administration cost was €264 (Appendix 1, ).
An alternative regimen frequently employed is docetaxel (100 mg/m2 q3w) combined with trastuzumab (8 mg/kg 1st dose and then 6 mg/kg q3w). The corresponding drug savings employing this regimen (40 weeks perspective – 13 courses – 90% DI) was estimated to €4306 for docetaxel and €5586 for trastuzumab, respectively ( and Appendix 1)
The average patient undergoes five CT scans and thoracic x-rays during treatment. The cost was calculated according to national prices Citation[24] and constituted €880 (Appendix 1).
Production gains were estimated, assuming 40% of women continuing in the workforce until 10 years after terminating adjuvant trastuzumab therapy and women in the comparative group later undergoing treatment for relapsing breast cancer to stay in the workforce for a median time of 3 years (including both time before relapse and time during therapy). Most of the benefit of adjuvant chemotherapy occurs during the first four years Citation[26]. In our calculations, relapse prevention was assumed to be equally distributed over the first six years after completing adjuvant chemotherapy. Details are shown in .
One of the second line regimens most frequently employed in metastatic setting in Norway is single-drug vinorelbine (30 mg/m2/w) and this regimen was included in our analysis.
When discounting, we calculated the second relapses equally distributed from the 2nd until the 8th year after initial treatment. Investigators have reported a median treatment duration employing vinorelbine in second line setting ranging between 10–23 weeks Citation[27–29]. Based on these studies, our own clinical experience and the fact that patients having a HER-2 positive tumor have a poor prognosis, we calculated second line chemotherapy lasting for a median time of 20 weeks. We anticipated two thirds of patients undergoing first line chemotherapy candidates for second line therapy. Three CT-scans and thoracic x-rays were included and constituted €353 (Appendix 1 and ).
In two of five Norwegian health regions, comprising about 50% of the population, general practise includes employing trastuzumab also in the second line setting. To clarify this situation, an alterative combining weekly vinorelbine with trastuzumab every 3 weeks (7 courses) was added. The trastuzumab cost was €2334.
Third line therapy implemented was capecitabine (1250 mg/m2 day 1–14/3w). We assumed two thirds of patients undergoing second line therapy to be candidates for third line therapy. The time perspective was 4–10 years and treatment duration 20 weeks ().
Adjuvant trastuzumab has so far had no influence on contra lateral breast cancer Citation[13]. Benefits related to a reduced number of new primary cancers were consequently not included. There may be improvement in the number of locoregional relapse, but the data are limited due to a short follow-up time. It was therefore excluded from the analysis.
Sensitivity analysis
In this survey, we performed a one-way analysis to establish the individual effect of each component. The sensitivity analysis included the following parameters: Discount rate: Several discount rates have been employed in international literature Citation[30–32]. In this sensitivity analysis, we employed a 0–5% range. Considering costs of out-patient treatment, administration, price of trastuzumab and travelling, production gains and life years gained, we varied each individual parameter with±25%.
Results
The results from the randomised trials Citation[12], Citation[13] indicate that the increased lifetime gains adding trastuzumab in adjuvant setting may improve the overall survival by 10–20%. This is illustrated in . The area between the FEC curve and the two suggested curves (10 or 20% improvement in OS) expresses the life years gained and is 1.1 and 2.19 discounted life years (LY) (1.5–3 undiscounted LY) gained per patient treated, respectively. The total cost per patient treated (savings exclusive) was calculated to €45 127–48 491, depending on whether production gains and travelling were taken into account. Including savings, the total cost was in the range €14 916–33 168, depending on discount rate (0 or 3%) and OS gain (10 or 20%, and ).
Table III. The table shows cost-effectiveness (C/E) ratios depending on key costing assumptions and 10% or 20% improved overall survival level. Effectiveness was calculated in life years gained (LYG) or QALYs. 3% discount rate was employed.
The CEA (3% d.r.) indicated cost per life year saved ranging between €8148 and €35 947 ( and ). Withdrawing trastuzumab from the use in metastatic setting raised the upper CEA-figure to €39 383. The 10% and 20% improved absolute overall survival rate as achieved at ten years follow-up and the 3% discount rate was employed calculating all the cost and effectiveness (life years gained-LYG) figures in .
The quality of life (Q = 0.8) () was based on the publication of Norum et al. Citation[33], the review by Mols and coworkers Citation[34] and the Harvard database (www.harvard.edu). Employing this figure, the corresponding cost per quality adjusted life year (QALY) was calculated in the range between €10 185 and €48 391 (). Specific quality of life figures during various treatment periods (adjuvant, relapse and palliative setting) among HER-2 positive breast cancer patients undergoing adjuvant trastuzumab therapy were not available. Furthermore, long time figures are absent and thus our QALY-calculations must be handled with care.
The alternative docetaxel-trastuzumab regimen followed by the vinorelbine-trastuzumab combination in second line metastatic setting ( – alternative regimens) did not have any significant influence on cost-effectiveness. The net health care resource [(C1-S1)/E, 20% improved OS and 3% discount rate] was only reduced from €15 341 to €14 594 per LYG.
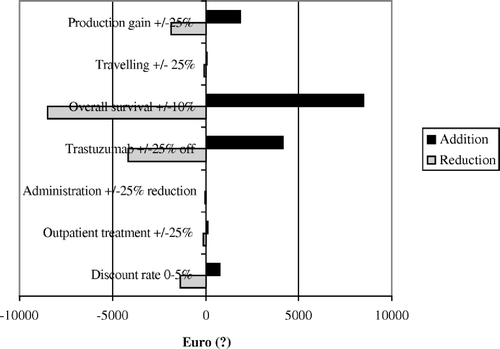
The univariate sensitivity analysis () revealed life years gained (LYG), the price of trastuzumab (+/ − 25%), production gain employed and discount rate (0–5%) to be the most important factor influencing cost-effectiveness. As long as trastuzumab is routinely used in the metastatic setting, LYG seems to be the only factor having significant impact on the cost-effectiveness. Norwegian authorities have indicated a cut-off limit of €54 000/LYG (NOK 425 000/LYG) Citation[35]. Employing this limit, adjuvant trastuzumab have to improve absolute 10-years overall survival by at least 8% to be cost-effective (net health care cost formulae; C1–S1).
Discussion
The major cost is trastuzumab, constituting 87% of the primary raised costs. The major economic benefits are chemotherapeutics avoided (29%) and production gains (60%). The amount saved on chemotherapy avoided is mainly due to the fact that trastuzumab is employed in Norway in first line metastatic setting. Thus, 19% of the savings in metastatic setting is directly related to trastuzumab therapy. The first column of shows how the cost figure, that form the basis for the C/E-calculations, depend on which cost – and cost-savings – items that are being taken into account. Given the methodological controversy related to the inclusion of production gains Citation[15], as well as the recommendations in guidelines in many countries (e.g. NICE) to ignore this item, the formula yielding the net societal cost figure of €17 844 is unlikely to be used. Since the production losses that arise during treatment are less uncertain than the production gains arising in the future, the formulae yielding €36 290 is more likely to be used. However, some might still ask for consistency on this matter and argue that if production gains are not being taken into account, neither should production losses. When using a societal economic perspective, it can be argued that also costs for added life-years should be implemented in the analysis. Drummond and colleagues Citation[15] argue that this is up to the analyst to determine the importance of this factor. Whereas this cost may be substantial in this setting, it should be kept in mind that the figure is difficult to suggest occurs decades later and have to be discounted. The cost may be somewhat balanced by the fact that costs of terminal care was not included in this survey. However, this fact should be kept in mind when thresholds are focused. Furthermore, given that travelling costs in our region would be higher than in most other countries (due to long distances), and that these cost items are relatively small in the current context, one could argue for a narrow costing approach that yield net health care costs per patient of €33 597 as the base case. However, the cost figures resulting from the alternative formulas are included.
While preliminary data on the use of trastuzumab in adjuvant chemotherapy are promising, the cost issue is a major concern. McLaren stated that the financial burden on the health system in the UK could be in the range of £400 000 per recurrence prevented Citation[36]. The one year cost of treatment per patient has been reported between $US 60 000 Citation[37] and $Cdn 35 –45 000 Citation[38]. Based on the latter cost figure, trastuzumab was not approved for funding in Ontario until after a substantial media frenzy Citation[39]. In England, a NHS primary care trust recently reversed its decision not to pay for trastuzumab in the adjuvant setting Citation[40].
Based on the preliminary results from three available international studies Citation[18], the Norwegian Breast Cancer Group (NBCG) has recommended trastuzumab as standard practice in adjuvant treatment of early breast cancer (www.nbcg.net). Employing our results, the coverage of trastuzumab in adjuvant setting can be justified on a cost-effectiveness basis.
The cost effectiveness results in our model-based study are consistent with the results from an American Citation[37] as well as a Canadian study Citation[41]. An overview Citation[42] including these studies and others was recently published. It concluded the cost per LYG in the range between €10 000 and 75 000, depending on method chosen and breast cancer patients focused. A 16% reduction in distant disease free survival at 4 years in the joint study Citation[12] may also support the assumption of an acquired benefit of about 20%. In his CEA, Hillner Citation[37] estimated an increase in overall survival exceeding one LYG at 13 years follow-up. This is in accordance with our suggestions (10% = 1 LYG at 15 years and 20% = 1 LYG at 10 years follow-up). A model based study by the Norwegian knowledge centre for the health services Citation[43] included HER2 positive women at the age of 50 years, data from the literature and the Cancer Registry of Norway (CRN) and suggested a survival gain of 2.7 years (from 77.5 to 80.2 yrs). The corresponding cost per LYG was calculated in the range between €9 000–19 000 (NOK 72 000–152 000), depending on discount rate employed (0–3%). A similar report from the Danish national board of health Citation[44] indicated a cost per LYG of DKK 78 475 (€10 516) (indirect costs exclusive). The life year gained employing trastuzumab in adjuvant setting was calculated 2.6 discounted life years and a 3% discount rate was employed.
However, as shown in our sensitivity analysis, the upfront Euro cost may clearly be reduced by lowering the costs related to trastuzumab therapy. The study of Joensuu et al. Citation[14] is such an example. They employed trastuzumab for only 9 weeks and revealed an improvement in 3 years distant disease free survival from 76% to 93% (p = 0.078) and a trend towards superior overall survival (p = 0.08). If future studies can document nine weekly cycles as effective as regimens lasting for one year, cost-effectiveness will surely be improved.
The regular use of trastuzumab in first line metastatic setting in Norway is one of the factors improving the cost-effectiveness of adjuvant trastuzumab. Interestingly, while the use of trastuzumab in metastatic setting has been indicated not cost-effective Citation[1], use of trastuzumab on this indication has been accepted by the Norwegian Health Authorities as well as the National Institute for Health and Clinical Excellence (NICE.) in the UK Citation[45].
Production gain is another important factor. At present 80% of Norwegian women aged fifty are in the working force according to Statistics Norway. At the age of 60, this percentage has dropped to 50%. The unemployment rate in Norway is 4.8%. Implementing the side effects of surgery, chemotherapy and radiotherapy, it is reasonable to suggest a lower percentage of women treated for breast cancer continuing in the work force. Based on all these factors, we believe our suggested guess of 40% returning to the workforce for a median time of 10 years being a reasonable figure. Other settings are illustrated in our sensitivity analysis. In this study, we did not implement cost of treatment in the terminal care setting nor did we include costs of hormonal therapy. HER2 positive metastatic breast cancer is known as a disease with poor prognosis. In this situation, chemotherapy is the most important therapeutic agent. We did not reveal sufficient data to implement these factors. However, if implemented, hormonal therapy may have caused a minor improvement of the cost-effectiveness figures. The magnitude of survival gain is the factor having the greatest influence on the cost-effectiveness figures. This was also concluded by Hillner Citation[37] and Neyt and colleagues Citation[46]. Based on the interim analysis from studies performed, a 10–20% improvement looks reasonable. However, it should be kept in mind that the patients entering clinical trials do not reflect average patients and the survival gain obtained in “real life” may be lower. It could be argued that different time horizons should be included in the sensitivity analysis. We believe this factor would make the analysis more complex and argue that it is taken care of within the 10–20% range employed.
Monoclonal antibodies are today the most costly drugs introduced in cancer therapy. Obviously, they will account for a significant amount of resources spent in cancer care in the near future. All of them have just been released on the market and are protected by patent regulations for several years ahead. A survey among American medical oncologists has revealed that they suggest a cut-off at US$ 300 000 per quality adjusted life year (QALY) Citation[47]. National as well as private health insurers and hospital owners have a clear interest in counteracting this trend of raising cost as long as their resources are limited.
In conclusion, trastuzumab looks cost-effective in adjuvant treatment of HER2 positive breast cancer in Norway employing a cut-off of €50 000/life year gained. At least 8% improvement in absolute ten years overall survival must be achieved.
The authors wish to thank Sidsel Kristiansen at the Pharmacy UNN, Anne-Torill Nordsletta at the Department of Finance UNN and Bjørn Naume at the Norwegian Radium Hospital for their support and collaboration in the project. The secretary assistance by Karen Krane Norum and the suggestions in the early process by professor Anthony Harris at the Centre for Health Economics, Business and Economics, Monash University, Clayton, Australia is also greatly appreciated. We are also thankful to Karianne Johansen at the Norwegian Health Service Research Centre in Oslo for her comments. The research grant from the Norwegian Cancer Union and the University Hospital of North Norway (UNN) are greatly appreciated.
References
- Norum J, Risberg T, Olsen JA. A monoclonal antibody against HER-2 (Trastuzumab) for metastatic breast cancer. A model based cost effectiveness analysis. Ann Oncol 2005; 16: 909–14
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer; correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–82
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–12
- King CR, Kraus MH, Aaronson SA. Amplification of a novel c-erbB–related gene in a human mammary carcinoma. Science 1985; 229: 974–6
- Seshadri R, Firgaira EA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. J Clin Oncol 1993; 11: 1936–42
- Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, et al. Her-2/neu expression in node negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res 1993; 53: 4960–70
- Piccart MJ, Di Leo A, Hamilton A. Her2: A predictive factor ready to use in the daily management of breast cancer patient?. Eur J Cancer 2002; 36: 1755–61
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First line Herceptin monotherapy in metastatic breast cancer. Oncology 2001; 61: 37–42
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line single-agent Herceptin (trastuzumab) in metastatic breast cancer. A preliminary report. Eur J Cancer 2001; 37: 25–9
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a.monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. NEJM 2001; 344: 783–92
- Marty M, Cognetti F, Maianinchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol 2005; 23: 4265–74
- Romond RH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. NEJM 2005; 353: 1673–84
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. NEJM 2005; 353: 1659–72
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. NEJM 2006; 354: 809–20
- Drummond, MF, O'Brian, B, Stoddart, GL, Torrance, GW. Methods for the economic evaluation of health care programmes. Oxford Medical Publications, 2nd ed. Oxford, New York, Toronto: Oxford University Press; 1997.
- Mullins CD, Ogilvie S. Emerging standardization in pharmacoeconomics. Clin Ther 1998; 20: 1194–202
- Norum J, Holtmon M. Adjuvant fluorouracil, epirubicin and cyclophosphamide in early breast cancer. Is it cost-effective?. Acta Oncol 2005; 44: 735–41
- De Laurentiis, M, Cancello, G, Zinno, L, Montagna, E, Malomi, M, Esposito, A, et al. Targeting HER2 as a therapeutic strategy for breast cancer: A paradigmatic shift of drug development in oncology. Ann Oncol 2005;16(Suppl. 4):iv7–iv13.
- Slamon, D, Eiermann, W, Robert, N, Pienkowski, T, Martin, M, Pawlicki, M, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients. BCIRG 006 study. San Antonio Breast Cancer Symposium. Abstr. 2005; 1069( www.abstracts.com/sabcs05): 2view
- Bonadonna, G, Moliterni, A, Zambetti, M, Daidone, MG, Pilotti, S, Gianni, L, et al. 30 years’ follow-up of randomised studies of adjuvant CMF in operable breast cancer. Cohort study. BMJ 2005;330:217, Epub.
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 2001; 98: 10869–74
- Norum J. Adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF) in breast cancer. Is it cost effective?. Acta Oncol 2000; 39: 33–9
- Severens JL, Milne RJ. Discounting health outcomes in economic evaluation; the ongoing debate. Value Health 2004; 7: 397–401
- The National Health Administration. The tariff of public out-patient treatment valid from January 1st 2005. Oslo: The National Health Administration; 2004.
- Department of Health and Care Services. Information about Diagnosis Related Groups 2005. Oslo; Department of Health and Care Services: 2005.
- Demicheli R, Miceli R, Moliterni A, Zambetti M, Hrushesky WJ, Retsky M, et al. Breast cancer recurrence dynamics following adjuvant CMF is consistent with tumor dormancy and mastectomy driven acceleration of the metastaic process. Ann Oncol 2005; 16: 1449–57
- Suzuki Y, Tokuda Y, Saito Y, Ohta M, Tajima T. Combination of trastuzumab and vinorelbine in metastatic breast cancer. Jpn J Clin Oncol 2003; 33: 514–7
- Nistico C, Garufi C, Milella M, Vaccaro A, D'Ottavio AM, Fabi A, et al. Weekly schedule of vinorelbine in pretreated breast cancer patients. Breast Cancer Res Treat 2000; 59: 223–9
- Verma S, Trudeau M, Dranitsaris G, Clemons M, Joy AA, MacKey JR. What is the best chemotherapy treatment option for anthracycline and taxane pretreated metastatic breast cancer. J Clin Oncol 2005; 23: 6260
- Belgian Society for Pharmacoepidemiology. A proposal for methodological guidelines for economic evaluation of pharmaceuticals. Brussels: Belgian Society for Pharmacoepidemiology (BESPE); 1995.
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russel LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996; 276: 1253–8
- Hjelmgren J, Berggren F, Andersson F. Health economic guidelines – similarities, differences and some implications. Value Health 2001; 4: 225–50
- Norum J, Olsen JA, Wist EA. Lumpectomy or mastectomy? Is breast conserving surgery too expensive?. Breast Cancer Res Treat 1997; 45: 7–14
- Mols F, Vingerhoets JJM, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors. A systematic review. Eur J Cancer 2005; 41: 2613–9
- Ministry of Finance. A guide to societal economic analysis. Oslo: Ministry of Finance; 2005. ( http://odin.dep.no/fin/norsk/dok/regelverk/retninglinjer/007021-990004/dok–bn.html)
- McLaren EH. Adjuvant trastuzumab for breast cancer. BMJ 2005; 331: 1203
- Hillner, BE.. Clinical and cost-effectiveness implications of the adjuvant trastuzumab in HER2+ breast cancer trials. San Antonio Breast Cancer Symposium. Abstr. 2005; 5040( www.abstracts.com/sabcs05): 2view
- Kondro W, Sibbald B. Patient demand and politics push Herceptin forward. CMAJ 2005; 173: 347–8
- Dent R, Clemons M. Adjuvant trastuzumab for breast cancer. We need to ensure that equity exists for access to effective and expensive treatments. BMJ 2005; 331: 1035–6
- Mayor S. Primary care trust reverse decision not to fund trastuzumab. BMJ 2005; 331: 1162
- Ragaz, J, Spinelli, JJ.. Cost-benefit estimates of adjuvant (Adj) trastuzumab (herceptin, H) for early breast cancer (BrCa). San Antonio Breast Cancer Symposium. Abstr. 2005; 2029( www.abstracts.com/sabcs05): 2view
- Norum J. The cost-effectiveness issue of adjuvant trastuzumab in early breast cancer. Expert Opin Pharmacother 2006; 7: 1617–25
- The Norwegian knowledge centre for the health services.Adjuvant therapy with trastuzumab in early stage breast cancer – An health economic analysis The Norwegian knowledge centre for the health services, Report no 18, Oslo, 2006 ( http://www.kunnskapssenteret.no/filer/Rapport1806_Herceptin_helseok.pdf)
- Danish national board of health. Trastuzumab (Herceptin) in adjuvant treatment of early breast cancer following surgery. Preliminary report. Med Teknol kræftlegemidler. 2005;1:1–21 ( http://www.sst.dk/publ/Publ2005/CEMTV/Laegemidler/Trastuzumab_syntese.pdf)
- Editorial. Herceptin and early breast cancer. A moment for caution. Lancet 2005; 366: 1673
- Neyt, M, Albrecht, J, Cocquyt, C. An economic evaluation of Herceptin in adjuvant setting. The breast cancer international research group 006 trial. Ann Oncol 2005; doi: 10.1093/annonc/mdj101.
- Nadler ES, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value?. Proc Am Soc Clin Oncol 2005; 23: a6011
Appendix 1: The appendix shows the calculations of costs.
Unit cost trastuzumab = NOK 6 592 (Pharmacy UNN, February 2006) = €822/unit.
Drug cost adjuvant trastuzumab=[cost/unit*units loading dose+(cost/unit*units standard dose*16 courses*dose intensity)]=[(€822*4.15)+(€822 * 3.15 * 16*0.94)] = €42 354.
Cardiac check ups=(tariff code 708ca + 202)*5=(NOK345 +185)*5 = €330
Out-patient clinic costs (National Health Administration): [(H06e + 201b)*17+ (H02*2 + H01*15) ]*dose intensity = NOK (801 + 245)*17 + 146*2 + 42*15)*0.94 = €2 192.
Regular follow-ups control group (two follow-ups the first year): (201b*2 + H02*2 = €98
Treatment of congestive heart failure = DRG127 * frequency = NOK37 300*0.005 = €23.
Production loss during adjuvant therapy=(€41 958*0.828)*17 days/260 days] = €2 272
Savings 1st line trastuzumab/paclitaxel in metastatic setting (20% improved OS 90% DI):
Trastuzumab: [(€822*2.15 + €822*1.15*35)*0.9*0.2] = €5 764.
Paclitaxel: [(€372*36*0.9*0.2] = €2 411.
Out-patient clinic cost: [(H06e + 201b)*36+ (H02*2 + H01*34)]*0.9*0.2 = NOK [(801 + 245)*36 + 146*2 + 42*34]*0.9*0.2 = NOK 7 088] = €884.
Pharmacy administration cost: (163.5*2*36*0.9*0.2/8.02) = €264
Alternative 1st line regimen (Docetaxel/trastuzumab, 13 courses–90%DI):
Docetaxel: [(€2163*13)*0.9*0.9*0.2] = €4 306
Trastuzumab: [((€822*4.15)+(€822*3.15*12))*0.9*0.9*0.2] = €5 586
CT scans and thoracic x-rays during 1st line treatment: [(tariff code 202 + PK001 + PK002 + x-ray PK113 + CT PK301)*5*0.9=(NOK185 + 136 + 136 + 113 + 999)*0.9*5] = €880.
CT-scans and thoracic x-rays during 2nd line treatment: [(tariff code 202 + PK001 + PK002 + x-ray PK113 + CT PK301)*3*0.9*0.67=(NOK 185 + 136 + 136 + 113 + 999)*1.8] = €353
Trastuzumab in 2nd line setting: [((€822*4.15)+(€822*3.15*6))*0.9*0.67*0.2] = €2 334.