Abstract
The aim of this study was to evaluate results of fractionated stereotactic radiotherapy (FSRT) in patients with residual or recurrent nasopharyngeal carcinoma (NPC) in terms of local progression-free (LPFS) and overall survival (OS) rate and complications after treatment. There were 32 residual or recurrent NPC patients treated with FSRT using linac-based radiosurgery system. Time from the previous radiotherapy to FSRT was 1–165 months (median, 15). Two patients were treated for the second and one for the third recurrence. Thirteen patients (40.6%) also received chemotherapy with FSRT. Tumor volume ranged from 6.2–215 cc (median, 44.4). Average FSRT dose was 17–59.4 Gy (median, 34.6) in 4–25 fractions (median,6) in 1–5.5 weeks (median, 3). Median follow-up time was 25.5(3–67) months. LPFS rate at 1 and 3 years after FSRT was 67.8% and 37.9%. OS rate at 1 and 3 years was 89.7% and 71.2%. If all patients who had tumor progression with no further follow-up were assumed dead, the OS rate at 1 and 3 years would be 75.0% and 37.9%. Univariate analysis showed better local tumor control in patients with tumor volume ≤100 cc (p=0.04) or in those without chemotherapy (p=0.0005). Only chemotherapy retained significance in multivariate analysis (hazard ratio 5.47, 95%CI 1.86–16.04). Eight patients (25%) had complications after FSRT, all grade 2–3 except 1 grade 4 with complete recovery.
Stereotactic Body Radiotherapy(SBRT) is one of the emerging novel technique in the radiotherapy community, as evident from the Third Acta Oncological Symposium on SBRT held in Copenhagen in June 2006 Citation[1]. Most presentations focused on lung and liver tumors. However this technique had been adopted for treatment of the skullbase and nasopharyngeal lesions for some time using the brain stereotactic radiosurgery/radiotherapy system. As nasopharyngeal carcinoma (NPC) is the most common head and neck cancer in Thailand Citation[2] and local recurrence still remained an important site of failure in patients with higher initial T stage, this article aimed to review a single institutional data regarding the results of fractionated stereotactic radiotherapy(FSRT) in patients with residual or recurrent NPC. Primary endpoint was local-progression-free survival rate (LPFS) and predictive factors. Secondary endpoints included overall survival rate (OS) and complications after treatment. The study was approved by the institutional review board.
Material and methods
Patient characteristics
From August 1998–March 2004 there were 32 consecutive residual or recurrent NPC patients treated with FSRT. Patients were included for FSRT when the lesion was considered not suitable for brachytherapy and the expected survival was longer than 6 months. Written informed consent was obtained before FSRT in every case. There were 23 males and 9 females. Age ranged from 27–84 years (median,46). All patients, except one with an unknown histology, had squamous cell carcinoma (2 WHO type 1, 15 WHO type2, 14 WHO type3). Twenty-one patients (65.6%) had initial T3 or 4 disease (AJCC 1992 & 1998). Initial treatment consisted of radiotherapy with chemotherapy in 25 patients (78.1%) and radiotherapy alone for the rest. Initial radiotherapy consisted of external beam radiotherapy (XRT) alone in 28 patients (87.5%) and XRT with brachytherapy in 4 (12.5%). Nasopharyngeal XRT dose ranged from 63–82 Gy (median,68.6) in 32–45 fractions (median,36). Time from the finish of initial radiotherapy to the detection of persistent / recurrent tumor was 1–165 (median,15) months. Histologic confirmation of persistent / recurrent tumor was achieved in 21 cases (65.6%). Two patients (6.3%) were treated for the second and one (3.1%) for the third recurrence. At the time of FSRT, three patients (9.4%) also had positive neck nodes and three (9.4%) had distant metastasis ( 2 at liver and 1 at bone). Karnofsky performance status at FSRT was 90 in 27 patients (84.4%), 80 in three patients (9.4%) and 70 in two (6.3%).
For the local treatment of the residual / recurrent tumor FSRT was given as the sole modality in 30 patients (93.8%). One patient had upfront XRT (30 Gy / 15 fractions) followed by FSRT and high dose chemotherapy with stem cell transplantation, the other one had surgical tumor removal before FSRT. Thirteen patients (40.6%) also received systemic chemotherapy. Chemotherapy regimen varied depending on the patient's condition and previous treatment, it was platinum-based in combination with 5-fluorouracil or paclitaxel or paclitaxel alone. Tumor volume at the time of FSRT was 6.2–215 cc (median,44.4). Three patients with positive neck nodes also received local neck XRT. Three patients with distant metastasis also received local treatment for the metastatic site (local XRT at the liver mass in one, wedge resection of the liver mass in one, and local XRT at the bony metastases in one).
After FSRT all patients were clinically evaluated every 1–6 months and imaging (CT or MRI) was performed at varying intervals depending on the patients’ symptoms. There were 24 patients (75%) who had follow-up imaging available for the analysis. The time of last follow-up imaging was 3–49 months (median,14) after FSRT.
FSRT technique
Treatments were performed using the linear accelerator base system (6 MV dedicated LINAC, Varian; with X-Knife planning system version 3 & 4, Radionics). The relocatable Gill-Thomas-Cosman frame was used for patient immobilization and target localization. Individual treatment planning was done based on a contrast-enhanced CT scan, 1.5-mm-slice thickness, with or without gadolinium-enhanced MRI. The prescribed dose could be 6 Gy x 6 fractions (2–3 weeks, Biologically Effective Dose-BED = 57.6 Gy) for small lesions away from critical structures (the brainstem, spinal cord, optic pathway, brain parenchyma), 2 Gy x 25 fractions (5 weeks, BED = 60 Gy) or 3 Gy x 10 fractions (2 weeks, BED = 39 Gy) for larger lesions near critical areas. The dose should cover at least 90% of the lesion volume (the GTV). Each physician then would adjust the dose at the margin to keep normal tissue dose to the range accepted. If the dose was considered not adequate chemotherapy would be given as an adjuvant treatment. In this series FSRT was given in 4–25 fractions (median,6) in 1–5.5 weeks (median,3). Number of isocenter used was 1–14 (median,7). Average FSRT dose given to the tumor was 17–59.4 Gy (median,34.6), minimum tumor dose was 0.2–45 Gy (median,15.9) and maximum tumor dose was 18.5–86.6 Gy (median,54.5). Total dose covering 90% of the tumor volume was converted into biologically effective dose (BED-D90) using the equation BED = total dose x (1 + d / α/β) where d = dose per fraction and α/β =10, no correction was made for tumor proliferation since 90% of the treatment was finished within 4 weeks. Total maximum dose to the tumor was converted the same way into BED-Dmax. In one patient with upfront XRT before FSRT the XRT dose was also converted and combined in the BED calculation. Median BED-D90 was 42.9 (11.3–95.2) Gy and median BED-Dmax was 97.6(49.1–197.5) Gy.
Data analysis
Primary endpoint, the LPFS, was defined as the time from the date of FSRT until local tumor progression (detected from clinical examination or imagings) or the date of last follow-up. Secondary endpoint, the OS, was defined as the time from the date of FSRT until the patient's death or the date of last follow-up. Adverse events after FSRT was graded according to the Common Terminology Criteria for Adverse Events version 3.0 Citation[3].
The survival probability was estimated by the Kaplan-Meier methods. Log-rank test was used to determine the association between various factors and the LPFS. Cox proportional hazard regression was used in the multivariate analysis to determine the independent factor associated with LPFS. All statistical analysis was performed using STATA software version 8 (STATA corporation, Texas, USA).
Results
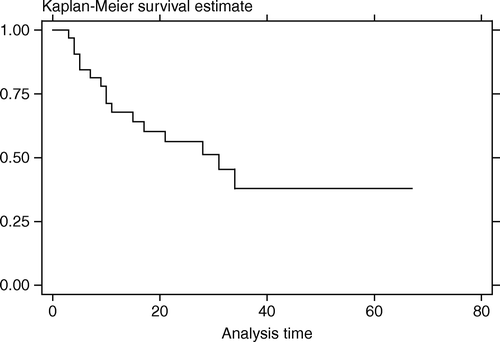
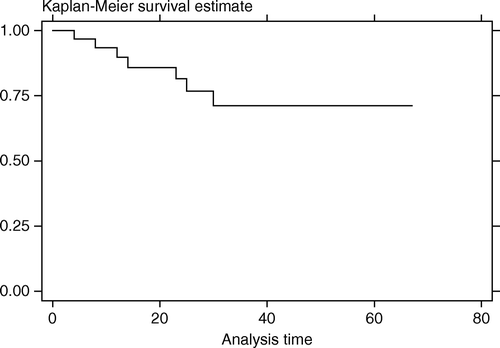
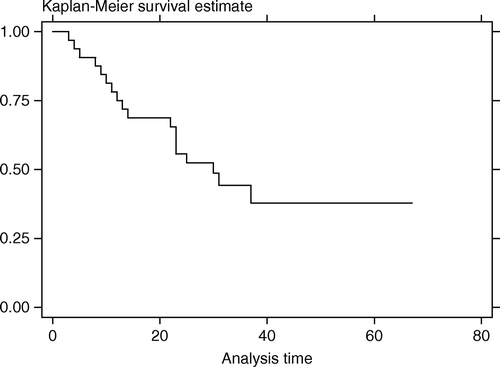
Follow-up time ranged from 3–67 months (median,25.5). At the time of last follow-up there were seven deaths, six from cancer (at 4–30 months after FSRT) and one from aspirated pneumonia with sepsis (at 8 months post FSRT). Local tumor progression after FSRT was detected in 16 patients at 3–34 months (median,10). Two patients developed nodal progression and three had distant metastasis after FSRT. The LPFS rate at 1 and 3 years after FSRT was 67.8% (95%CI, 48.4–81.3%) and 37.9% (95%CI, 17.6–58.2%). Median LPFS time was 31 months (). The OS rate at 1 and 3 years after FSRT was 89.7% (95%CI, 71.3–96.6%) and 71.2% (95%CI, 48.1–85.5%). Median survival time was not reached (). Most patients maintained regular follow-up visit post FSRT until disease progression was detected, after which many sought supportive treatment elsewhere near their habitat and the clinical details could not be retrieved. So we tried making an assumption of death right after the last visit for patients who were lost to follow-up after the detection of tumor progression, and the OS rate at 1 and 3 years dropped to 75.0% (95%CI, 56.2–86.6%) and 44.2% (95%CI, 26.0–61.0%) with the median survival time of 30 months ().
Figure 3. Overall survival curve assuming death for all patients with tumor progression who were lost to follow-up (months)
showed baseline characteristics of the patients who had local control after FSRT compared to those with local tumor progression. Five factors including time from the previous radiotherapy (≤12 VS >12 months), BED-D90 (≤40 VS >40 Gy), BED-DMAX (≤90 VS >90 Gy), tumor volume (≤100 VS > 100 cc), and receiving chemotherapy with FSRT (no VS yes) were included in the univariate analysis to detect the association with LPFS. As shown in , only tumor volume and chemotherapy were significantly associated with the local control. Better local tumor control was seen in patients with tumor volume ≤100 cc (p = 0.04) or in those without chemotherapy (p = 0.0005). Multivariate analysis showed that chemotherapy was the only significant factor, patients receiving chemotherapy had worse LPFS with the hazard ratio of 5.47 (95% CI, 1.86–16.04).
Table I. Baseline characteristics of the patients who had local tumor control after FSRT compared to those with local tumor progression.
Table II. Univariate analysis exploring factors associated with local-progression-free survival.
Eight patients (25%) had adverse events after FSRT. There were four patients with more trismus (grade 2), four patients with more decreased hearing (3 grade 2 and 1 grade 3 requiring hearing aid), one patient with transient ischemic brain symptoms >24 hr. (grade 4) with complete recovery, one patient with dysphagia and hoarseness from cranial nerves dysfunction (grade 2), and one patient with headache and earache (grade2).
Discussion
Evidences have shown that retreatment of locally recurrent nasopharyngeal cancer was feasible, patients were relieved from local suffering symptoms and some experienced a long term survival. Several techniques have been reported including reirradiation using external beam and/or brachytherapy. Wang Citation[4] et al. showed that high-dose reirradiation was required if long-term control was the goal, in their series none receiving dose < 60 Gy survived at 5 years compared to 45% 5-year survival rate in the group treated with higher dose. However patients receiving high dose reirradiation were subjected to high rates of severe complications such as brain necrosis, cervical myelopathy, swallowing dysfunction, mandibular or soft tissue necrosis Citation[5], thus significantly compromised the quality of life gained. In a large series of 654 recurrent NPC patients treated with reirradiation reported by Lee and associates Citation[6], 5-year local salvage rate was 23% and the complication-free rate was 52%. They suggested a combination of teletherapy and brachytherapy when feasible. Although widely available and a familiar technique limitation remains for brachytherapy in treating irregular leions infiltrating high into the skullbase and brain.
Stereotactic radiosurgery was originated as the mean to give single high dose radiation intracranially to the small volume, usually by a diameter not exceeding 3 cm. Later development enabled fractionated treatment more suitable for malignancy or larger lesions abutting critical structures. Many institutes adopted this technique to treat skullbase lesions including the nasopharyngeal tumors. Both single dose radiosurgery and fractionated stereotactic radiotherapy were used as the sole modality or in combination with XRT or brachytherapy for the locally persistent / recurrent tumors or as a booster dose after conventional treatment Citation[7–18]. At our hospital the linac- based radiosurgery system was installed in 1997 and we started treating nasopharyngeal cancer cases in 1998. Our preliminary results Citation[19] showed that the crude local control rate was much different in the boost group (controlled 8/8 cases, follow-up time 2–33 months) compared to the retreatment group (controlled 6/11 cases, follow-up time 4–19 months). This study was conducted to explore the results from longer follow-up of the retreatment group.
From the univariate analysis, patients who had the detection of persistent / recurrent tumor within 1 year from previous radiotherapy and patients receiving higher FSRT dose (BED-D90 and BED-Dmax) tended to have better local control but statistical significance was not reached. Larger tumor volume (>100 cc) resulted in poorer local control (p = 0.04). However patients receiving chemotherapy also had worse local control (p = 0.0005), the result remained significance in multivariate analysis controlling for tumor volume (hazard ratio 5.47, 95% CI, 1.86–16.04). However the wide 95% CI reflected a small sample size of this study. It was not possible to conclude that chemotherapy led to the poor local control because there were selection factors which could lead to the worse outcome. The patients who received chemotherapy had larger tumor volume (median 46 cc VS 35.8 cc in the group without chemotherapy) and also had a larger proportion of those with disease outside the nasopharynx (30.8% VS 10.5%). Although having distant disease at the time of FSRT could not directly explain the poor local control but it might indicate the more aggressive behavior of the residual/recurrent tumor. To address the role of chemotherapy in this setting further randomized trials are necessary.
.The assumption of death right after the last visit for patients with tumor progression after FSRT and didn't have further follow-up data was the way we used to estimate the OS. So the assumed OS rate at 1 and 3 years of 75.0% and 44.2% with the median survival time of 30 months would be worse than in reality since many lived for some period longer after tumor progression before they succumbed to the disease. Due to the incomplete end point data, the analysis to assess factors associated with OS was not performed in this study.
The complication-free survival was not reported in this study because almost all patients had at least one type of complication from the previous treatment. The crude complication rate of 25% expressed the new symptoms after FSRT. There was no fatal hemorrhage after FSRT in the local controlled cases as previously reported Citation[11] but there were five patients with uncontrolled tumor who experienced tumor bleeding during the end stage. The optimal dose-fractionation of FSRT has yet to be defined as the lower dose per fraction might decrease severe late complications but requires more resources and probably would result in decreased tumor control probability.
With variations in the patients’ characteristics, tumor volume, radiation dose-fractionation, and the use of systemic chemotherapy the only conclusion this series could contribute was that this technique was feasible for nasopharyngeal reirradiation with minimal toxicity. Many questions remain that need further studies including the way to detect early residual/recurrent disease, optimal dose-fractionation, the benefit of systemic chemotherapy and the role of this technique in the era of all the modern techniques like the intensity modulated radiotherapy or the image-guided radiotherapy.
Acknowledgements
The authors thank Mr. Prasert Assavaprathuangkul for his help with the database and Ms. Sasivimol Rattanasiri for her kind contribution in the data analysis review. This study did not receive financial support and there are no conflicts of interest.
References
- Grau C, Hoyer M, Lindegaard J, Overgaard J. The emerging evidence for stereotactic body radiotherapy. Acta Oncol 2006; 45: 771–4
- Sumitsawan Y, Srisukho S. Nasopharynx. Cancer in Thailand, H Sriplung, S Sontipong, N Martin, S Wiangnon, V Vootiprux, A Cheirsilpa, C Kanchanabat, T Khuhaprema. Bangkok Medical Publisher, Bangkok 2003; 22–4
- Commom Terminology Criteria for Adverse Events v3.0. Available from: http://ctep.cancer.gov/reporting/ctc_v30.html
- Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma-treatment techniques and results. Int J Radiat Oncol Biol Phys 1987; 13: 953–6
- Pryzant RM, Wendt CD, Delclos L, Peters LJ. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys 1992; 22: 941–7
- Lee AWM, Foo W, Law SCK, Poon YF, Sze WM, SK O, et al. Reirradiation for recurrent nasopharyngeal carcinoma: factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys 1997; 38: 43–52
- Kaplan ID, Adler JR, Hicks WL, Fee WE, Giffinet DR. Radiosurgery for palliation of skull base recurrence from head and neck cancers. Cancer 1992; 70: 1980–4
- Buatti JM, Friedman WA, Bova FJ, Mendenhall WM. Linac radiosurgery for locally recurrent nasopharyngeal carcinoma: Rationale and technique. Head Neck 1995; 17: 14–9
- Cmelak AJ, Cox RS, Adler JR, Fee WE, Goffinet DR. Radiosurgery for skull base malignancies and nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1997; 37: 997–1003
- Orecchia R, Redda MG, Ragona R, Nassisi D, Fossa BJ, Zurrida S, et al. Results of hypofractionated stereotactic re-irradiation on 13 locally recurrent nasopharyngeal carcinomas. Radiother Oncol 1999; 53: 23–8
- Ahn YC, Lee KC, Kim DY, Huh SJ, Yeo IH, Lim DH, et al. Fractionated stereotactic radiotherapy for extracranial head and neck tumors. Int J Radiat Oncol Biol Phys 2000; 48: 501–5
- Xiao JP, Xu GZ, Miao YJ. Fractionated stereotactic radiosurgery for 50 patients with recurrent or residual nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2001; 51: 164–70
- Chen HJ, Leung SW, Su CY. Linear accelerator based radiosurgery as a salvage treatment for skull base and intracranial invasion of recurrent nasopharyngeal carcinomas. Am J Clin Oncol 2001; 24: 255–8
- Pai PC, Chuang CC, Wei KC, Tsang NM, Tseng CK, Chang CN. Stereotactic radiosurgery for locally recurrent nasopharyngeal carcinoma. Head Neck 2002; 24: 748–53
- Chua DTT, Sham JST, Kwong PWK, Hung KN, Leung LHT. Linear accelerator-based stereotactic radiosurgery for limited, locally persistent, and recurrent nasopharyngeal carcinoma: efficacy and complications. Int J Radiat Oncol Biol Phys 2003; 56: 177–83
- Le QT, Tate D, Koong A, Gibbs IC, Chang SD, Adler JR, et al. Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2003; 56: 1046–54
- Yau TK, Sze WM, Lee WM, Yeung MW, Leung KC, Hung WM, et al. Effectiveness of brachytherapy and fractionated stereotactic radiotherapy boost for persistent nasopharyngeal carcinoma. Head Neck 2004; 26: 1024–30
- Low JS, Chua ET, Gao F, Wee JT. Stereotactic radiosurgery plus intracavitary irradiation in the salvage of nasopharyngeal carcinoma. Head Neck 2006; 28: 321–9
- Dhanachai M, Kraiphibul P, Pochanugool L, Dangprasert S, Sitathanee C, Laothamatas J, . Stereotactic radiotherapy in nasopharyngeal carcinoma–preliminary results. Radiosurgery, D Kondziolka, M McDermott, J Regis, R Smee, K Takakura, et al. Karger, Basel 2002; 162–6