Abstract
We have previously described patient-reported outcomes of late side effects induced by conventional external beam radiotherapy (EBRT), 4 and 8 years after treatment, in 181 patients with localized prostate cancer compared with 141 age-matched controls. In the present study, we compare bowel side effects 15 years after EBRT with the same controls, and with the results of our previous 4-year and 8-year follow-ups. Of the 181 patients and 141 controls at the 4-year follow-up, 45 patients (25%) and 79 controls (56%) were still alive at the 15-year follow-up. Bowel symptoms were assessed using the symptom-specific questionnaire Prostate Cancer Symptom Scale (PCSS), which was sent to these 45 patients and 79 age-matched controls with a mean follow-up time of 15 years (162–197 months) after EBRT. The answer frequency was 64% in the patient group and 52% in the control group. The mean age was 78 years in both groups. At the 15-year follow-up, 39% of the patients and 84% of the controls reported no bowel problems (p <0.001), while 16% of the patients and 0% of the controls reported “Quite a few/many” problems with mucus in the stools (p <0.001). “Quite a bit/much” stool leakage was reported by 20% of the patients at the 15-year follow-up, in comparison to 4% of the patients at the 4-year follow-up (ns). The proportion of patients reporting late bowel symptoms was unchanged 15 years after EBRT in comparison to the 4-year follow-up. Increased bowel symptoms were seen in patients in comparison to the age-matched controls.
There are two radical therapeutic options for localized prostate cancer (LPC); radical prostatectomy (RP) and radiotherapy (external and/or brachytherapy). These options have undergone significant refinement during the past decade, with many new technical improvements producing higher than 95% cancer-specific survival at 5 years after primary treatment Citation[1–3]. However, there are still no large randomized trials that show a significantly superior outcome for either treatment option. Since these treatment options are in general associated with different types and levels of side effects, detailed information on the prevalence of both acute and late toxicity is of great importance for patients as well as physicians.
Radiation therapy is correlated with a higher percentage of bowel symptoms than RP. Bowel symptoms include rectal bleeding, mucus in stools, faecal leakage, diarrhoea, and more frequent stools Citation[4–6]. Many of the rectal symptoms seem to improve over time Citation[7–9].
Higher dose improves the prostate specific antigen (PSA), progression-free survival, and prolongation of time to metastasis; and the improvement of the EBRT technique from conventional to three-dimensional conformal technique, intensity-modulated radiation therapy (IMRT), and protons has made it possible to escalate the dose above 70 Gy Citation[10–14].
With the conventional 4-field box technique, and doses <70 Gy, only a few per cent of patients suffer serious late side effects Citation[15], Citation[16]. However, most studies have been based on physicians’ estimation of the major side effects, and there is only limited information about patient-reported outcomes of late side effects measured with self-assessment questionnaires, especially regarding very late morbidity (≥ 8 years after primary treatment) Citation[17].
In our efforts to better understand the patient's perception of very late side effects, we have previously reported on side effects 4 and 8 years after EBRT in LPC patients treated with conventional techniqueC:\GetARef\Refs\15 r bowel.ref #3;, self-assessed with questionnaires and compared with an age-matched control population Citation[18].
We now prospectively revisit the same cohort of both LPC survivors and the age-matched controls 7 years later, at a mean of 15 years after primary treatment, to describe and compare the morbidity changes over time as well as in comparison to a prostate cancer free population of age-matched controls using the same self-administered questionnaire.
To our knowledge, no study using a patient-evaluated self-assessment questionnaire for evaluation of side effects after EBRT for LPC has been performed with both a prospective comparison of late (4/8 years) and very late (15 years) side effects and a comparison with a cohort of age-matched prostate cancer free population.
Methods
Patient and control populations
Between 1986 and the middle of 1989, 284 LPC patients received EBRT to the pelvis with a curative intention at the Department of Oncology Radiotherapy Unit, Umeå, Sweden. From the primary group of 284 patients, we excluded 89 patients who according to the Swedish population register were dead at the date of the submission of the first questionnaire (1991), patients with distant metastases, and patients who received a total dose of less than 60 Gy (). The first self-assessed questionnaire (4-year follow-up) was sent out in April 1991 (patient group) and November 1991 (controls), giving a mean follow-up time from the start of radiotherapy of 4 years (range 24–56 months) Citation[6].
Table I. Data collection procedure for prostate cancer (PC) patients and controlsin the 15-year follow-up.
The second questionnaire was sent out in May 1995 to the same patient group, giving a mean follow-up of 8 years from the start of radiotherapy (range 6–9 years). The questionnaire was also sent to the control group at the same time Citation[18].
The present study is a 15-year prospective follow-up of the same patient and control groups. The present questionnaires were sent out in December 2002, giving a mean follow-up of 15 years from the start of radiotherapy (). The patient group was also compared with the same age-matched control group as included in both the 4-year and 8-year follow-ups, of men from the same region as the population of treated patients with PC () Citation[6], Citation[18].
Table II. Characteristics of the patients and controls.
Treatment technique
Computer tomography (CT) planned EBRT was given with the conventional 4-field box technique, 5 days a week, 2 Gy per fraction, and with an average total dose of 64.8 Gy. The total dose of treatment was based on a CRE value of 18.5 (mean 18.3; range 18–19). Large treatment volume was extended cranially to the sacral promontory, caudally to the ischial tuberosity, laterally to the medial walls of the pelvis, ventrally to the symphysis, and dorsally to sacrum including the whole rectum resulting in an anterior-posterior field size of approximately 12×16 cm and a lateral field size of approximately 10×16 cm.
Two treatment techniques were used: one with shrinkage field (type A), and one where a large treatment volume was given up to full dose (type B).
Type A
For treatment of localized PC (mostly T0diff-T2); large treatment volume receiving 50 Gy, thereafter a reduction including prostate only with a 2 cm margin. The shrinkage field size was 9×9×9 cm (anterior-posterior-lateral).
Type B
For treatment of locally advanced PC (mostly positive lymph nodes and/or T3, T4, and all Grade 3), including large treatment volume up to full dose.
One patient had undergone a prostatectomy 3 months before EBRT, but was given treatment type A with a total dose of 65.3 Gy.
Instrument for evaluation of bowel side effects
Bowel problems were evaluated with a validated Prostate Cancer Symptom Scale self-assessment questionnaire (PCSS) Citation[6], Citation[18–22]. The questionnaire contains four main categories: general symptoms, bladder symptoms, bowel symptoms, and sexual function. Evaluation of bladder symptoms and sexual function does not form part of the present study, and will be reported separately.
The PCSS questionnaire uses a modified linear analogue scale, with response boxes containing numerical values between 0 and 10, where 0 = “no problem/very good function” and 10 = “many problems/very bad function”. The patients were asked to evaluate their symptoms during the previous week. Additional questions request a written answer; these questions are not summarized or reported in the present study.
Statistical methods
Mean values were calculated for all items. The original numerical scale was also transformed to a 4-point verbal scale, with 0–1 being transformed to “No problems”, 2–4 to “A little problems”, 5–7 to “Quite a bit problems”, and 8–10 to “Very much problems”.
The non-parametric Mann-Whitney test was used to evaluate changes over time and differences between the patient and control groups. All patients included in the present follow-up answered all three questionnaires (4-, 8-, and 15-year). Three of the controls did not answer the 8-year follow-up questionnaire, but did answer the 15-year follow-up questionnaire; despite this, these three controls were included in the analyses in the present study. Correlation coefficients were calculated according to Pearson and considered significant when p < 0.05.
All analyses were performed using SPSS statistical software, version 14.0.
Permission to perform the study was given by the Ethics Committee of the Faculty of Medicine at Umeå University, Sweden.
Results
Patients and controls
Between the 4-year and 15-year follow-up, 136 of 181 patients (75%) and 62 of 141 controls (44%) had died (). The answer frequency was 64% (29 of 45) in the patient group and 52% (41 of 79) in the age-matched control group (). Four men in the control group were diagnosed with prostate cancer between the 8-year and 15-year follow-up, and were therefore excluded from the analysis, leaving 37 controls to be analyzed in the present study (). Patient characteristics are presented in .
Two of the patients had also been diagnosed with another cancer before the diagnosis of prostate cancer; one with rectal cancer (diagnosed in 1986, 2 years before PC) and one with bladder cancer (diagnosed in 1969, 30 years before PC).
Bowel problems
In the patient group, there was no difference in bowel symptoms between 4 and 15 years after treatment. In the control group, there was a decrease in blood in stools, mucus, and bowel problems in general between 4 and 8 years and also between 4 and 15 years (), but no difference in problems between 8 and 15 years. However, all bowel symptoms were higher in the patient group in comparison to the controls at all three follow-ups ().
Table III. Bowel symptoms at the 4-year, 8-year, and 15-year follow-ups.
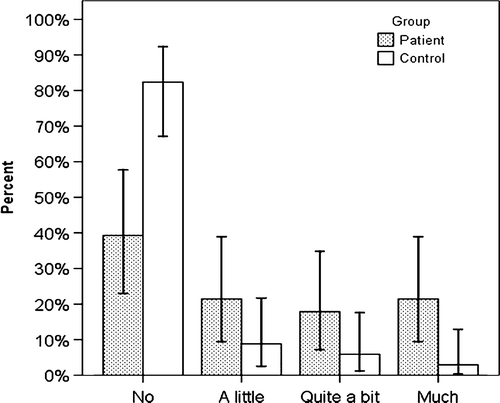
At the 15-year follow-up, 39% of the patients and 84% of the controls reported “no” bowel problems (p < 0.001; ), while 16% of the patients and 0% of the controls reported “Quite a bit/much” problems with mucus in the stools (p < 0.001). Also at the 15-year follow-up, 32% of the patients had mucus in the stools, with 18% having “a little”, 11% “quite a bit of”, and 4% “very much” mucus. One of the controls reported “a little” mucus in the stools, while the rest did not report any mucus (p < 0.001).
Figure 1. Error bars (95% CI) showing the patients’ (n = 29) and controls’ (n = 37) answers to the question about bowel problems in general at the 15-year follow-up.
When comparing the patients over time, 20% reported “quite a bit /much” stool leakage at 15 years in comparison to 4% at the 4-year follow-up (ns).
Seventy-one per cent of the patients had no blood in stools 15 years after EBRT, while 21% reported “a little”, 4% “quite a bit”, and 4% “very much”. None of the controls reported blood in the stools (p < 0.001).
Overall, 7% of the patients and 46% of the controls were “Very content”, 36%/26% were “Content”, 14%/17% felt “Satisfactory”, 21%/3% had “Mixed feelings”, 11%/9% were “Displeased”, and 11%/0% felt “Unhappy” about living the rest of their lives with their bowel problems as they are today, respectively.
Treatment type
The treatment type (A or B) did not have any impact on the related bowel problems (). One patient was treated with post-operative EBRT. No significant difference regarding bowel problems could be calculated in comparison with the other EBRT patients. However, this patient had high scores regarding almost all types of problems (ns).
Table IV. Bowel symptoms at the 15-year follow-up, stratified by treatment type and presence or absence of hormonal treatment.
Hormonal treatment
Fourteen of the 29 patients (48%) had received hormonal treatment at the time of the 15-year follow-up. Ten of them had undergone surgical castration. There were no differences in bowel problems at 15 years after treatment between those who had and had not received hormonal treatment ().
Age
There was no difference in age between patients (78 years) and controls (77 years) at the 15-year follow-up. Age had no significant impact on the reported bowel problems at the time for follow-up in either the patient or control groups.
Smoking
There were no differences in the level of bowel problems in general between smokers (including ex-smokers) and those who had never smoked, in either the patient group (10 smokers/ex-smokers, 18 non-smokers) or the control group (22 smokers/ex-smokers, 14 non-smokers) (). There were also no differences when the individual bowel problems were analyzed separately ().
Table V. Bowel symptoms at the 15-year follow-up, stratified by smoking behaviour and the presence of diabetes.
Diabetes
Four of the patients (14%) and nine of the controls (24%) reported that they had diabetes at the time of the 15-year follow-up (). The analysis of the individual bowel problems showed no differences between those with diabetes and those without, in either the patients or the controls ().
Medicines
Sixteen of the 29 patients were using warfarin or acetylsalicylacid at the time of the 15-year follow-up. However, no difference regarding rectal bleeding could be seen when comparing those with (mean = 1.3) or without (mean = 1.0) this type of medicine (p = 0.8219).
Abdominal surgery
An earlier surgical operation in the abdomen was reported by a total of seven patients, including the patient who had undergone post-operative EBRT. No data regarding this information was registered from the controls. No difference was seen when comparing those with or without earlier abdominal surgery; however, there was a trend towards more symptoms in the surgical group (ns).
Missing case analyze
To help validate the results, we compared the characteristics of the respondents (n = 70) with those of the non-respondents (n = 54; ). Mean age differed between respondents and non-respondents in both the patient group (78 years and 83 years respectively, p = 0.014) and the control group (78 years, inclusive of the four controls with PC, and 82 years respectively, p = 0.003). When comparing the profile of bowel problems at 8-year follow-up of the respondents with the non-respondents no difference in problems could be measured in the patient or control groups ().
Table VI. Bowel symptoms at the 8-year follow-up stratified by respondents and non-respondents on the 15-year follow-up.
Discussion
With continued very long prospective patient-evaluated follow-up we have shown that the reported bowel toxicity remains relatively stable between 4 and 15 years after EBRT. We have also observed a clear difference with increased bowel problems in the treated LPC patients in comparison with age-matched controls followed with the same time schedule. It is notable that the bowel problems in the controls seem to have improved over time.
Many studies have shown significantly more prevalent bowel dysfunction after radiotherapy (external and/or brachytherapy) treatment than after radical prostectomy. However, to our knowledge, no previous longitudinal study of this type with this very long follow-up time of 15 years has been published.
In the present study we could not detect any change in bowel problems over time after 4 years post-treatment. Others have reported that bowel symptoms continue to increase or evolve 2 years after 3-D CRT and brachytherapy Citation[23–25]. However, there is limited information on patient-assessed very late bowel toxicity, and no evidence regarding symptom progress after 8 years post-treatment.
The present study does have some limitations. The small number of patients (n = 29) means that the results are difficult to generalize to a prostate cancer population, and also that the statistical analysis is uncertain. The answer frequency was relatively low in both groups (52–64%), probably because of the advanced age of the subjects. There was a significant difference in age between respondents and non-respondents in both the patient and the control group; the respondents were 4–5 years younger then the non-respondents. However, when calculating the impact of age on the reported bowel problems no correlations was found.
The non-responding patients also seemed to be those with more advanced disease. Thirty-seven per cent of the non-respondents had T3-T4 tumours (10% of the responders). This may mean that our study shows the panorama of symptoms in the healthiest group of patients. While this may also be the case in the control group, it is more difficult to come to a definite conclusion since we did not register any information from the controls other than that provided by the questions included in the questionnaire.
However, no difference in the bowel problems profile could be measured between the respondents and non-respondents when comparing the reported problems at 8-year both in patient and control groups.
Another important limitation is that these patients were treated with the “old” conventional large 4-field box technique, with a total dose of only 65 Gy, which is rather uncommon today. The information we have gained is still valuable, due to the lack of very long-term follow-up studies in the literature. However, since the side-effect patterns after ongoing technique developments in the treatment of prostate cancer the needs for very long evaluation of side effects are important.
The lack of baseline (pre-treatment) bowel function data makes it difficult to compare the real changes from pre-treatment values and the late side effects at 15 years. At the time the patients were treated, in the late 1980s, it was not usual for studies to include the collection of self-assessed baseline data, and so our follow-ups do not report such data. However, the strength of this study is that our findings provide a basis of information regarding radiation-induced changes in bowel toxicity, since the same information was collected from an age-matched control population at the same time intervals.
This report provides information from patients treated at a single institution in the northern part of Sweden. Regional, individual, and technical differences may have had an impact on the results; however, the control population was recruited from the same regional area. A study design with baseline (pre-treatment) information is important as well comparison with other treatment options e.g. radical prostectomy. However, such prospective multicenter study in two regions in Sweden is now ongoing.
The total prevalence of rectal bleeding (blood in stools) at 15 years in the present study was 29%, a figure which is within the range of 19–43% described in the literature Citation[5], Citation[6], Citation[8], Citation[9], Citation[26–30]
None of the controls reported blood in stools in the present 15-year follow-up. This finding of low frequency of blood in stools was also reported by Henningsohn et al. Citation[31] in a general Stockholm population.
The total frequency of faecal incontinence at 15 years was 57% in the patient group. The major part (36%) of this was reported as “a little”, while 11% of the patients reported “quite a bit of”, and 11% “very much” faecal incontinence. This high proportion, with more than 50% of patients suffering from faecal incontinence, was also reported by Geinitz et al. Citation[26] in patients up to 8 years after 3D-CRT and also by Fokdal et al. Citation[27], who reported a value of 58%, but with shorter follow-up time of 12 months.
The faecal incontinence of about 2–8% in our control group correlates very well with the findings of Adolfsson et al. Citation[32] in a general Stockholm population.
In summary, this study presents data from a very long follow-up of 15 years after EBRT with patient-assessed questionnaires. The results correspond very well to previously-reported results.
Conclusion
Bowel symptoms after conventional EBRT are frequent. Increased bowel symptoms were seen in patients in comparison to the age-matched controls. The proportion of patients reporting late bowel symptoms was unchanged 15 years after EBRT in comparison to the 4-year follow-up showing that evaluation 4 years after treatment may predict the level of very late side effects. This report emphasizes the importance of continuing with prospective collection of patient self-assessed evaluation, including baseline data with a multicentre design, and incorporating comparison of different treatment options. Such work will provide more information about outcomes of toxicity to both clinicians and patients, resulting in realistic expectations of side effects. It is also important to verify that new treatment modalities, with smaller margins, lead to less long-term toxicity.
Acknowledgements
This investigation was supported by grants from the Swedish Cancer Society and the Research Foundation of the Department of Oncology, University of Umeå.
References
- Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352: 1977–84
- Nilsson S, Norlén BJ, Widmark A. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol 2004; 43: 316–81
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 2001; 28: 555–65
- Goldner G, Zimmermann F, Feldmann H, Glocker S, Wachter-Gerstner N, Geinitz H, et al. 3-D conformal radiotherapy of localized prostate cancer: A subgroup analysis of rectoscopic findings prior to radiotherapy and acute/late rectal side effects. Radiother Oncol 2006; 78: 36–40
- Koper PC, Jansen P, van Putten W, van Os M, Wijnmaalen AJ, Lebesque JV, et al. Gastro-intestinal and genito-urinary morbidity after 3D conformal radiotherapy of prostate cancer: Observations of a randomized trial. Radiother Oncol 2004; 73: 1–9
- Widmark A, Fransson P, Tavelin B. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer 1994; 74: 2520–32
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst 2004; 96: 1358–67
- Hanlon AL, Watkins Bruner D, Peter R, Hanks GE. Quality of life study in prostate cancer patients treated with three-dimensional conformal radiation therapy: Comparing late bowel and bladder quality of life symptoms to that of the normal population. Int J Radiat Oncol Biol Phys 2001; 49: 51–9
- Little DJ, Kuban DA, Levy LB, Zagars GK, Pollack A. Quality-of-life questionnaire results 2 and 3 years after radiotherapy for prostate cancer in a randomized dose-escalation study. Urology 2003; 62: 707–13
- Dearnaley DP, Hall E, Lawrence D, Huddart RA, Eeles R, Nutting CM, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005; 92: 488–98
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53: 1097–105
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005; 294: 1233–9
- Zelefsky MJ, Fuks Z, Leibel SA. Intensity-modulated radiation therapy for prostate cancer. Semin Radiat Oncol 2002; 12: 229–37
- Lee AK, Frank SJ. Update on radiation therapy in prostate cancer. Hematol Oncol Clin North Am 2006; 20: 857–78
- Amdur RJ, Parsons JT, Fitzgerald LT, Million RR. Adenocarcinoma of the prostate treated with external-beam radiation therapy: 5-Year minimum follow-up. Radiother Oncol 1990; 18: 235–46
- Hanks GE, Diamond JJ, Krall JM, Martz KL, Kramer S. A ten year follow-up of 682 patients treated for prostate cancer with radiation therapy in the United States. Int J Radiat Oncol Biol Phys 1987; 13: 499–505
- Penson DF, Litwin MS, Aaronson NK. Health related quality of life in men with prostate cancer. J Urol 2003; 169: 1653–61
- Fransson P, Widmark A. Late side effects unchanged 4–8 years after radiotherapy for prostate carcinoma: A comparison with age-matched controls. Cancer 1999; 85: 678–88
- Fransson P, Bergström P, Löfroth PO, Widmark A. Five-year prospective patient evaluation of bladder and bowel symptoms after dose-escalated radiotherapy for prostate cancer with the BeamCath(R) technique. Int J Radiat Oncol Biol Phys 2006; 66: 430–8
- Fransson P, Bergstrom P, Lofroth PO, Widmark A. Prospective evaluation of urinary and intestinal side effects after BeamCath((R)) stereotactic dose-escalated radiotherapy of prostate cancer. Radiother Oncol 2002; 63: 239–48
- Fransson P, Damber JE, Tomic R, Modig H, Nyberg G, Widmark A. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer 2001; 92: 3111–9
- Fransson P, Tavelin B, Widmark A. Reliability and responsiveness of a prostate cancer questionnaire for radiotherapy-induced side effects. Support Care Cancer 2001; 9: 187–98
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002; 20: 557–66
- Potosky A, Legler J, Albertsen P, Stanford J, Gilliland F, Hamilton A, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000; 92: 1582–92
- Talcott JA, Manola J, Clark JA, Kaplan I, Beard CJ, Mitchell SP, et al. Time course and predictors of symptoms after primary prostate cancer therapy. J Clin Oncol 2003; 21: 3979–86
- Geinitz H, Zimmermann FB, Thamm R, Erber C, Müller T, Keller M, et al. Late rectal symptoms and quality of life after conformal radiation therapy for prostate cancer. Radiother Oncol 2006; 79: 341–7
- Fokdal L, Honoré H, Høyer M, von der Maase H. Dose-volume histograms associated to long-term colorectal functions in patients receiving pelvic radiotherapy. Radiother Oncol 2005; 74: 203–10
- Christie D, Denham J, Steigler A, Lamb D, Turner S, Mameghan H, et al. Delayed rectal and urinary symptomatology in patients treated for prostate cancer by radiotherapy with or without short term neo-adjuvant androgen deprivation. Radiother Oncol 2005; 77: 117–25
- Nguyen LN, Pollack A, Zagars GK. Late effects after radiotherapy for prostate cancer in a randomized dose-response study: Results of a self-assessment questionnaire. Urology 1998; 51: 991–7
- Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: The patient's perspective. Urology 1996; 47: 387–94
- Henningsohn L, Wijkström H, Dickman PW, Bergmark K, Steineck G. Distressful symptoms after radical cystectomy with urinary diversion for urinary bladder cancer: A Swedish population-based study. Eur Urol 2001; 40: 151–62
- Adolfsson J, Helgason AR, Dickman P, Steineck G. Urinary and bowel symptoms in men with and without prostate cancer: Results from an observational study in the Stockholm area. Eur Urol 1998; 33: 11–6