Abstract
Plasminogen Activator Inhibitor type-1 (PAI-1) is involved in tumor invasion and progression. High levels of PAI-1 are associated with poor prognosis in breast cancer, and PAI-1 has been shown to play a role in angiogenic processes. Since estimates of tumor angiogenesis may predict poor prognosis we studied the relationship between PAI-1 and estimates of angiogenesis in breast cancer. Tumor tissue specimens from 438 breast cancer patients were included. Median follow-up was 10.3 years. Protein levels of PAI-1 were measured using an ELISA. Angiogenesis scores were performed using a Chalkley grid. Median PAI-1 level was 0.70 ng/mg protein (range, 0 – 90 ng/mg protein) and median Chalkley count was 5.00 (range, 2.67 – 12.00). Chalkley counts were not correlated with PAI-1. In univariate analysis both increasing PAI-1 and increasing Chalkley counts evaluated as continuous parameters were significantly associated with poor disease-specific survival with RR 1.04 (95% CI 1.02 – 1.07) (p<0.0001) and RR 1.11 (95% CI 1.01 – 1.22) (p=0.04), respectively. High tertiles of PAI-1 were borderline significantly correlated with poor disease-specific survival (p=0.06), whereas high tertiles of Chalkley counts were significantly associated with poor disease-specific survival (p=0.004). Combining low/low versus high/high tertiles of Chalkley counts and PAI-1 showed actuarial 10-year survival rates of 82% versus 52% (p=0.004). High N-stage (p<0.0001), grade (p<0.0001) and increasing levels of PAI-1 (p=0.009) were independent markers of death from breast cancer. This study confirms high PAI-1 or high Chalkley counts as markers of poor prognosis in breast cancer patients, and suggests that the prognostic impact of PAI-1 is independent of its supposed involvement in tumor angiogenesis.
Plasminogen Activator Inhibitor-1 (PAI-1) is a glycoprotein with two active segments; one segment inhibits uPA and tPA and thereby the activation of plasminogen into plasmin the latter being directly involved in extracellular proteolysis. PAI-1 may thus act as a key regulator of protease induced tumor invasion and microenvironment remodelling. The other segment inhibits the interaction between vitronectin and different cell-surface-associated molecules (e.g. uPA/uPAR complexes and integrins) thereby influencing cellular adhesion and migration Citation[1], Citation[2]. In several experimental murine studies PAI-1 has been shown to play a critical role in tumor angiogenesis Citation[2], but the data are not consistent. One explanation for this discrepancy may be that the influence of PAI-1 on angiogenesis is dependent on tumor type, experimental setting, and content of tumor PAI-1 versus host PAI-1. However, there are several studies on the prognostic value of protein levels of PAI-1 as measured by ELISA in breast cancer Citation[3–6]. A meta- and pooled analysis including 8 377 breast cancer patients unequivocally demonstrated the significant association between tumor tissue PAI-1 levels and breast cancer survival Citation[7]. This inverse association between survival and PAI-1 tumor tissue levels strongly suggest that PAI-1 may have yet other tumor promoting functions. Indeed, we have recently published that PAI-1 may inhibit tumor cell apoptosis thereby facilitating tumor progression Citation[8], Citation[9].
Estimates of tumor angiogenesis have been extensively investigated in breast cancer and most studies show high vascular densities closely associated with poor disease-specific survival Citation[10], Citation[11].The possible association between PAI-1 and estimates of angiogenesis has only been investigated in few smaller studies where these parameters were found to be independent Citation[12], Citation[13].
This study presents data on PAI-1 protein levels measured by ELISA and estimates of tumor angiogenesis in 438 tumors from patients diagnosed with early breast cancer. A possible interaction between these parameters is explored.
Material and methods
Patients and tumor samples
Tumor material was collected from 438 consecutive patients diagnosed with early breast cancer where adequate tumor material was available. Patients were included in the study from January 1990 to 1994 and fulfilled the following criteria: having primary unilateral breast carcinoma with no evidence of metastasis; availability of complete clinical, histopathological and biological information; having no other malignancies; having received radical surgical therapy. Median age was 57 years (range, 29 – 89 years), 300 patients were postmenopausal, and the distribution of T1, T2 and T3 classification was 168, 235 and 35 cases, respectively. Ductal carcinoma was found in 370 tumors with malignancy grades (using WHO malignancy grading system) I, II and III seen in 84, 151, and 135 of these tumors, respectively. Three hundred and fifteen patients had estrogen receptor (ER) positive tumors. Forty eight percent of the patients were node-negative.
Treatment
Treatment of the patients was described in detail previously Citation[11]. Locoregional treatment consisted of surgery, total mastectomy or lumpectomy combined with adjuvant radiotherapy (48 Gy/24 fx., 5 fx. weekly) to residual breast. The systemic adjuvant treatment was given according to the national Danish treatment policy described by The Danish Breast Cancer Cooperative Group (DBCG 89 protocols). In this way systemic therapy was offered to all women at increased risk of recurrence (at that time node-positive, tumor size > 5 cm, or ductal carcinoma grade II/III), thus these women were allocated to one of three different protocols: DBCG 89b (premenopausal with ER+ tumor) was a randomization between CMF (inj. cyclophosphamide 600 mg/m2+methotrexate 40 mg/m2+5-fluorouracil 600 mg/m2) and castration, DBCG 89c (postmenopausal with ER+ tumor or unknown tumor) was a randomization among tamoxifen (30 mg daily) for 1 year, 2 years or 6 months followed by Megace for 6 months, DBCG 89d (administered to premenopausal ER- or unknown receptor status, or malignancy grade II/III, and to postmenopausal patients < 70 years with ER- tumors) was a randomization among CMF, CEF (inj. cyclophosphamide 600 mg/m2+epirubicin 60 mg/m2+5-fluorouracil 600 mg/m2), CMF + pamidronate (tabl. 150 mg x 2 daily for 4 years), and CEF + pamidronate administered nine times at intervals of 3 weeks.
Follow-up
According to the DBCG recommendations all patients were followed by clinical examination every third month the first year, then twice yearly until the fifth year, then once yearly from the sixth to the tenth year. After this period, information about recurrence was achieved from the clinical records at the treating hospital. Some older patients were followed by their general practitioner and referred to hospital if recurrence was suspected. All patients were followed from the date of operation and at least for 10 years or until death. Furthermore, all records were revised and survival status assured by contact to the National Population Register and in case of death we contacted the Danish Cancer Register and the National Causes of Death Register. In this way complete information was available regarding disease-specific survival (DSS). Information regarding first relapse was also available, however, with shorter follow-up.
PAI-1 ELISA
The method used has been described in detail previously Citation[3]. In short, cytosol extracts originally prepared for oestrogen receptor analyses using a standard procedure, including precooling in liquid nitrogen, pulverization with a Micro-Dismembrator, and extraction at 4°C with a buffer consisting of 10 mM K2HPO4/KH2PO4, 1.5 mM K2EDTA, 10 mM monothioglycerol, 10% glycerol (v/v), and 10 mM sodium molybdate (pH 7.5), followed by centrifugation at 105 000 x g for 1 h at 4°C were used. The supernatants were stored at -70°C. PAI-1 was determined using a sandwich ELISA kit (Monozyme, Horsholm, Denmark) with monoclonal catching and detecting antibodies. This assay recognizes active PAI-1, latent PAI-1, and PAI-1 complexed with uPA.
Immunohistochemical staining and microvessel quantification
This method has been described in detail previously Citation[11]. Briefly, 4 µm sections from formalin-fixed, paraffin-embedded tumor blocks were microwaved in 10 mM citrate buffer and immunostained with anti-CD34 monoclonal antibody (clone QB-END 10, Immunotech, France) diluted 1:50. Primary antibody was detected with LSAB (K681, Dako, Denmark) and visualized with DAB.
At x40-100 magnification the area of the tumor with highest microvascular density was found, and at x200 magnification estimates of angiogenesis were counted using a Chalkley eyepiece graticule placed in the ocular. The mean of three individual hot spots was reported to characterize the vascular density of the individual tumor. The evaluation was done by one person (B.V.O.), and the reproducibility was acceptable Citation[11].
Statistical analysis
The correlations among tertiles of the PAI-1 protein levels and the Chalkley counts, respectively, and other known ordinal clinicopathological parameters were investigated by Spearman′s rho, whereas a χ2 test was used to investigate the correlations among tertiles of PAI-1 protein levels and Chalkley counts, respectively, and nominal parameters, i.e. age and histopathology. Survival functions were made according to the Kaplan-Meier method and the differences among the survival curves were calculated with a log-rank test with a test for trend. All time estimates were made using the date of primary operation as initial value. A multivariate Cox proportional hazards regression analysis was used to investigate the prognostic value of the different parameters regarding death from cancer. The Cox analysis was stratified according to ductal versus non-ductal histology since non-ductal carcinomas had no malignancy grade. The statistical method was backward Likelihood Ratio. A Cox analysis was performed with data combined in the Nottingham Prognostic Index (NPI, i.e. maximum tumor size in centimeters times 0.2 + tumor malignancy grade + lymph node status (1 = node negative, 2 = 1 to 3 positive nodes, 3 = more than 3 positive nodes)). NPI was separated in three groups according to established guidelines (http://poptop.hypermart.net/brcanpi.html). Univariate and multivariate analyses were performed using the SPSS 13.00 program package. All p-values were based on two-sided testing and the level of statistical significance was 5%.
Results
PAI-1 protein levels, Chalkley estimates, and clinicopathological parameters
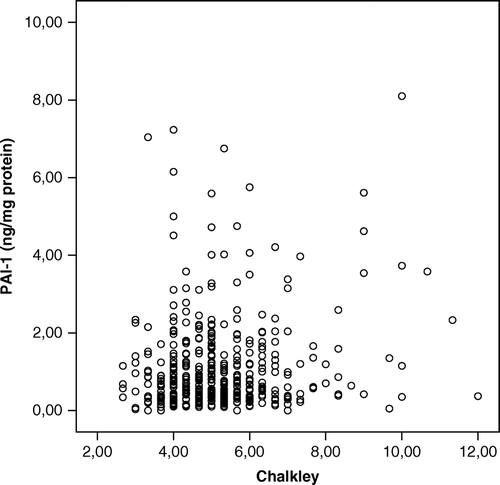
Median PAI-1 was 0.70 ng/mg protein (range, 0 – 90 ng/mg protein), and the median Chalkley count was 5.00 (range 2.00 – 12.00). Chalkley counts were not correlated with PAI-1 protein levels (r = 0.05; p = 0.23), and , bottom. highlights the distribution of PAI-1 levels and Chalkley counts in tertiles according to classical clinicopathological parameters. Both high Chalkley counts and high PAI-1 levels were significantly associated with known markers of poor prognosis i.e. high malignancy grade and negative oestrogen receptor status. Furthermore, high Chalkley counts were associated with large tumor size.
Table I. Patient and tumor characteristics in 438 early breast cancer patients stratified by PAI-1 protein levels and Chalkley counts divided into tertiles.
PAI-1 protein levels, Chalkley estimates and prognosis
Median follow-up time was 10.3 years (range 0 – 14.5 years), and at analysis 219 patients (50%) were alive and followed with a median of 11.3 years (range 10.0 – 13.8 years). One hundred and seventy six patients (40%) had died from breast cancer with a median follow-up of 3.9 years (range 0.4 – 14.5 years), whereas 43 patients (10%) had died from other causes. Regarding disease-free survival (DFS) 206 patients (47%) had experienced a relapse at a median follow-up of 2.3 years (range 0 – 14.5 years). The majority of relapses were distant metastases (83%).
shows the 10-year disease-specific survival rates for known classical clinicopathological parameters, PAI-1 protein levels and Chalkley counts in tertiles. Significant information on poor DSS was found in large tumor size, high malignancy grade in ductal carcinoma, negative oestrogen receptor status, presence of positive lymph nodes, high Chalkley counts, and borderline significant information was seen with high PAI-1 levels. Age and menopausal status were not significant prognostic markers.
Table II. 10-year disease-specific survival probability in 438 patients diagnosed with early breast cancer.
In univariate analysis increasing PAI-1 evaluated as a continuous parameter was significantly associated with poor disease-specific survival (DSS) with a RR 1.04 (95% CI 1.02 – 1.07) (p < 0.0001). After separating PAI-1 in tertiles the highest tertile of PAI-1 was not significantly associated with poor DSS RR 1.19 (0.99 – 1.43) (p = 0.06), as graphically illustrated in . However, when separating the patients into node-negative and node-positive, high tertiles of PAI-1 were significantly associated with poor DSS among node-negative patients RR 1.66 (1.15 – 2.39) (p = 0.006) but not among node-positive patients (p = 0.72) ( and ). The 10-year disease-specific survival rates among the node-negative patients were for the low to the high PAI-1 tertiles 87±4%, 80±5%, and 68±6% respectively, and among the node-positive patients the corresponding figures for the low to the high PAI-1 tertiles 50±6%, 40±6% and 49±6%, respectively.
Figure 2. Disease-specific survival in 438 patients diagnosed with early breast cancer stratified by PAI-1 protein levels divided into tertiles.
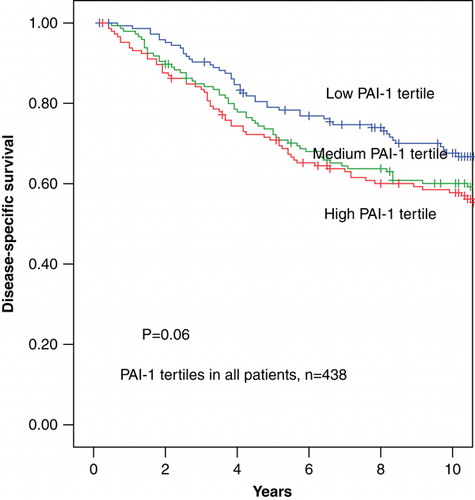
Figure 3. Disease-specific survival in 210 patients diagnosed with node-negative early breast cancer stratified by PAI-1 protein levels divided into tertiles.
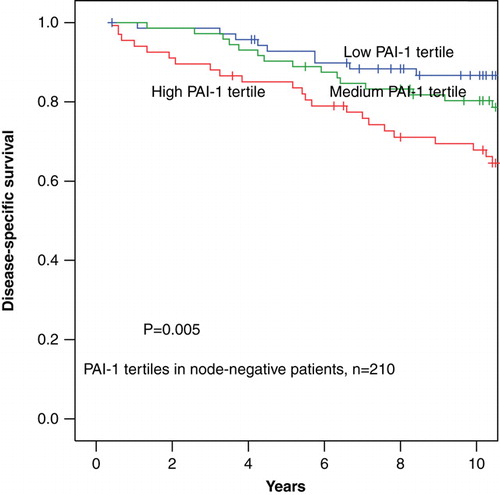
Figure 4. Disease-specific survival in 228 patients diagnosed with node-positive early breast cancer stratified by PAI-1 protein levels divided into tertiles.
Increasing Chalkley counts evaluated both as continuous parameters and in tertiles were significantly associated with poor DSS in the whole group RR 1.11 (95% CI 1.01 – 1.22) (p = 0.04), and RR 1.29 (1.08 – 1.16) (p = 0.005), respectively, and among node-positive patients RR 1.44 (1.16 – 1.78) (p = 0.001), however, not among node-negative patients (p = 0.66) (not shown).
To further investigate the association between PAI-1 protein levels and Chalkley counts we evaluated the distribution of malignancy grade and survival rates at 10 years follow-up as shown in . From a close relationship among PAI-1 levels in tertiles and malignancy grade appeared, thus in the distribution of malignancy grade according to the combination of PAI-1 and Chalkley tertiles is listed. From this table it appears that the combination of low PAI-1 and low Chalkley count is dominated by malignancy grade I, whereas high malignancy grades are seen for combinations of high PAI-1 and high Chalkley counts, respectively. The 10-year survival rates identified patient groups with different outcome as illustrated by colours in . The group with the low/low PAI-1/Chalkley tertile (blue color) had a different survival as compared to the group of high/high, medium/high and high/medium PAI-1/Chalkley (red color), respectively, versus the rest (green color). This is also shown in illustrating the highly significant different survival of these groups. In the tumors have been separated into node-negative and node-positive to illustrate that the interaction between PAI-1 and Chalkley tertiles is much more prominent among the node-positive as compared to the node-negative patients. The survival rates among the PAI-1 tertiles are almost similar when the Chalkley score is low, however, when the Chalkley counts increase the survival rates decrease dramatically.
Figure 5. The distribution of the patients according to the values of PAI-1 and Chalkley. The colours refer to . Blue: low/low PAI-1 and Chalkley, red: high/high, medium/high, high/medium PAI-1/Chalkley, green: the rest. The disease-specific survival rates at 10 years were: 81±5% (blue), 63±3% (green), 54±4% /red).
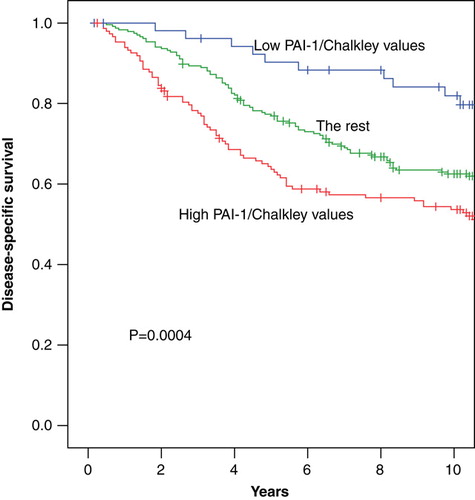
Table III. The distribution of PAI-1 protein levels divided into tertiles according to Chalkley counts divided into tertiles. The 10-year survival rates±s.e. (%) are reported. In bold the distribution of malignancy grade I/II/III for ductal carcinomas, respectively, for all patients. Please, see Results for an explanation of the background for the table.
A similar picture appeared when evaluating the prognostic influence of PAI-1 and Chalkley counts on DFS. PAI-1 as a continuous parameter was significantly associated with DFS in the whole group with RR 1.03 (95% CI 1.02 – 1.05) (p < 0.0001), and among node-negative patients with a RR 1.04 (95% CI 1.02 – 1.06) (p < 0.0001), whereas PAI-1 was not significantly associated with poor DFS in node-positive patients. Continuous Chalkley counts were significantly related to poor DFS in the whole group with a RR 1.13 (95% CI 1.03 – 1.23) (p = 0.008) and among node-positive patients with a RR 1.29 (95% CI 1.15 – 1.45) (p < 0.0001), whilst they were not significant in node-negative patients.
In multivariate analysis using death from breast cancer as endpoint including all patients, high number of positive lymph nodes, high malignancy grade in ductal carcinomas, and increasing PAI-1 levels (continuous variable) were all independent prognostic markers, whereas no prognostic information was gained from oestrogen receptor status, menopausal status, tumor size and Chalkley counts. However, in the group of patients with node-positive disease the following characteristics were all significantly independent markers of poor outcome: >3 positive lymph nodes, high malignancy grade, negative oestrogen receptor status, and increasing Chalkley count. No significant prognostic information was obtained by PAI-1 levels in this patient group. Among node-negative patients, PAI-1 levels were the most informative marker of death with a RR 1.04 (95% CI. 1.02 – 1.07) meaning that the risk of death from breast cancer increased with 4% with each ng/mg increase in PAI-1 (). If the multivariate analysis was made with a combined score of PAI-1/Chalkley count, a significantly increased risk of death was found when comparing the low/low to the high/high PAI-1/Chalkley, respectively, (). Moreover, a Cox analysis including the Nottingham Prognostic Index, menopausal status, oestrogen receptor status and the tertiles of PAI-1 and Chalkley, respectively, was made. In that analysis only NPI was an independent prognostic marker of cancer-death with relative risks of 1, 3.59 (95% CI, 2.40 – 5.38), and 7.11 (95% CI, 4.27 – 11.82), respectively, for the three groups of NPI.
Table IV. Cox multivariate analysis; endpoint death from breast cancer.
Discussion
This study showed that PAI-1 protein levels were not correlated with tumor tissue vessel density as estimated by Chalkley counts. Both parameters were associated with patient prognosis. However, combining PAI-1 with Chalkley counts separated the patients into groups with even more significantly different prognosis. Patients with tumors having low/low PAI-1/Chalkley counts had a 10-year survival rate of 82±7% as compared to 52±7% for tumors with high/high PAI-1/Chalkley counts. In a Cox multivariate analysis including both PAI-1 levels and Chalkley counts together with established prognostic markers, increasing PAI-1 levels but not Chalkley counts were identified as an independent marker of breast cancer death.
The ELISA used in the present study was performed on frozen tumor tissues, whilst angiogenesis estimates were evaluated using formalin-fixed, paraffin-embedded tissue sections. Thus, the estimates of these parameters were made in two different areas of the same primary tumor. Tumor heterogeneity is a well known fact. Therefore, it has to be considered that the observed lack of association between PAI-1 protein levels and Chalkley counts could be due to the fact that they were evaluated in two different areas of the same tumor.
This study confirms that patients with tumors with low PAI-1 protein content have a significantly better DSS as compared with patients with tumors having high PAI-1 protein levels Citation[7]. This was the case in the whole study group, but after separating the patients into node-negative and node-positive, it was seen, that the significance of PAI-1 level in predicting prognosis was found only among the node-negative patients. From it appears that a high proportion of the tumors with low levels of PAI-1 were also classified as malignancy grade I, however, both PAI-1 and malignancy grade were independent prognostic markers in the multivariate analysis. Also, it has to be mentioned that node-positive patients received adjuvant therapy, whereas node-negative patients with no other risk factors did not, thus lack of systemic adjuvant therapy in node-negative patients could also be an explanation. However, in the study by Look et al. Citation[7] PAI-1 was identified as a strong prognostic marker in both node-negative and node-positive patients in 8 377 patients treated with both adjuvant radiation and chemotherapy. In this study Chalkley counts were independent markers of poor prognosis in node-positive patients only, thus the Chalkley counts could only be used to separate those patients with an extremely poor prognosis from those patients with a poor prognosis. The Chalkley counts were not able to separate the node-negative patients into groups with different prognosis.
Only few studies have investigated the association between PAI-1 protein levels and vascular scores. In one study Citation[12] including 228 patients and another Citation[13] including 136 patients, no correlations between PAI-1 protein level and Chalkely counts were found. However, none of the studies combined PAI-1 and Chalkley scores in relation to patient prognosis.
The biological rationale for PAI-1 being involved in tumor development has been investigated in several cell culture studies and animal tumor models as reviewed lately by Durand et al. Citation[2]. PAI-1 has been implicated in cell adhesion, migration and invasiveness through its interactions with uPA, vitronectin and/or uPA-uPAR-complexes. A decade ago it was established that high levels of uPA in primary tumors were associated with poor outcome Citation[14]. It was therefore an unexpected finding that high levels of the uPA inhibitor PAI-1 as measured by ELISA were also closely related to poor prognosis of cancer patients Citation[15]. Some explanations for this observation may be suggested; e.g. PAI-1 may protect components in the extracellular matrix from degradation by uPA thereby maintaining a skeleton for cancer cells to migrate upon. This hypothesis is supported by the demonstration of PAI-1 mRNA and protein mainly being present in stromal cells (endothelial cells and myofibroblasts) in breast cancer tissue Citation[16]. The hypothesis has gained further support from experimental studies which suggest a significant role of PAI-1 in the development of tumor angiogenesis (see below). However, it can still not be ruled out if the association between PAI-1 and poor prognosis is a surrogate for aggressive tumors without PAI-1 having an independent biological role.
The possible influence of PAI-1 on angiogenesis has been considered in several studies, many of them using PAI-1 gene-deficient (PAI-1-/-) mice. In PAI-1-/- mice malignant keratinocytes transplanted into skin failed to stimulate angiogenesis Citation[17], and delayed formation of new vessels into Matrigel implants in PAI-1-/- mice has also been demonstrated Citation[18]. In another study on PAI-1-/- mice subretinal choroidal angiogenesis induced by laser photocoagulation was prevented, however, after PAI-1 gene expression was normalized in these mice normal choroidal angiogenesis was also re-established Citation[19]. The supposed causal role of PAI-1 in corneal angiogenesis has lately been questioned in another study Citation[20], thus a consensus has not been reached. The cellular source and concentration of PAI-1 is very important as demonstrated lately Citation[21]. It was found that host PAI-1 was more important for tumor growth than tumor PAI-1, and moreover that host PAI-1 at physiologic level promoted angiogenesis whereas supraphysiological levels either by host cells or by tumor cells inhibited angiogenesis.
Experimental results have recently suggested that PAI-1 is a strong protector of apoptosis in cancer cells Citation[8], Citation[9], Citation[22]. In particular, it was shown that apoptosis induced by chemotherapy, e.g. adriamycin could be enhanced by deleting the PAI-1 gene from the cells. Thus, an apoptosis protective role of PAI-1 could be yet another explanation to the observed association between high PAI-1 tumor tissue levels and poor breast cancer patient prognosis.
In conclusion, the finding of PAI-1 protein level being independent of Chalkley estimates may have several different explanations. PAI-1 has been implicated in the angiogenic process in different experimental settings although the exact mechanisms are still unclear. The influence of PAI-1 on angiogenesis in human cancer in situ may be completely different. Furthermore, PAI-1 may be only transiently involved in angiogenesis during tumor growth and thus more associated with vessel formation than vessel density. Finally, PAI-1 is involved in many other aspects than angiogenesis of tumor growth as reviewed by Andreasen et al. Citation[15], Citation[23]. The problem of risk assessment in breast cancer patients remains a critical issue. Further studies are needed to guide clinicians in choosing the right therapy for the right patient. At the present time different anti-tumor targeted therapy strategies are under clinical investigation and a thorough understanding of tumor biology is essential to optimize these strategies.
References
- Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res 1985; 44: 139–266
- Durand MKV, Bødker JS, Christensen A, Dupont DM, Hansen M, Jensen JK, et al. Plasminogen activator inhibitor-I and tumour growth, invasion, and metastasis. Thromb Haemost 2004; 91: 438–49
- Grondahl-Hansen J, Christensen IJ, Rosenquist C, Brunner N, Mouridsen HT, Dano K, et al. High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res 1993; 53: 2513–21
- Janicke F, Schmitt M, Graeff H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost 1991; 17: 303–12
- Harbeck N, Schmitt M, Kates RE, Kiechle M, Zemzoum I, Janicke F, et al. Clinical utility of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 determination in primary breast cancer tissue for individualized therapy concepts. Clin Breast Cancer 2002; 3: 196–200
- Knoop A, Andreasen PA, Andersen JA, Hansen S, Laenkholm AV, Simonsen AC, et al. Prognostic significance of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in primary breast cancer. Br J Cancer 1998; 77: 932–40
- Look M, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, et al. Pooled analysis of prognostic impact of uPA and PAI-1 in breast cancer patients. Thromb Haemost 2003; 90: 538–48
- Romer MU, Due AK, Larsen K, Hofland KF, Christensen IJ, Buhl-Jensen P, et al. Indication of a role of plasminogen activator inhibitor type I in protecting murine fibrosarcoma cells against apoptosis. Thromb Haemost 2005; 94: 859–66
- Lademann U, Romer MU, Jensen PB, Hofland KF, Larsen L, Christensen IJ, et al. Malignant transformation of wild-type but not plasminogen activator inhibitor-1 gene-deficient fibroblasts decreases cellular sensitivity to chemotherapy-mediated apoptosis. Eur J Cancer 2005; 41: 1095–100
- Hansen S, Grabau DA, Sorensen FB, Bak M, Vach W, Rose C. The prognostic value of angiogenesis by Chalkley counting in a confirmatory study design on 836 breast cancer patients. Clin Cancer Res 2000; 6: 139–46
- Offersen BV, Sorensen FB, Yilmaz M, Knoop A, Overgaard J. Chalkley estimates of angiogenesis in early breast carcinoma: Relevance to prognosis. Acta Oncol 2002; 41: 695–703
- Hansen S, Overgaard J, Rose C, Knoop A, Laenkholm AV, Andersen J, et al. Independent prognostic value of angiogenesis and the level of plasminogen activator inhibitor type 1 in breast cancer patients. Br J Cancer 2003; 88: 102–8
- Fox SB, Taylor M, Grondahl-Hansen J, Kakolyris S, Gatter KC, Harris AL. Plasminogen activator inhibitor-1 as a measure of vascular remodelling in breast cancer. J Pathol 2001; 195: 236–43
- Duffy MJ, Reilly D, O'Sullivan C, O'Higgins N, Fennelly JJ, Andreasen P. Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res 1990; 50: 6827–9
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer 1997; 72: 1–22
- Offersen BV, Nielsen BS, Hoyer-Hansen G, Rank F, Hamilton-Dutoit S, Overgaard J, et al. The myofibroblast is the predominant plasminogen activator inhibitor-1-expressing cell type in human breast carcinomas. Am J Pathol 2003; 163: 1887–99
- Bajou K, Noel A, Gerard RD, Brunner N, Holst-Hansen C, Skobe M, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 1998; 4: 923–8
- McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westrick RJ, et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem 2001; 276: 33964–8
- Lambert V, Munaut C, Noel A, Frankenne F, Bajou K, Gerard R, et al. Influence of plasminogen activator inhibitor type 1 on choroidal neovascularization. FASEB J 2001; 15: 1021–7
- Vogten JM, Reijerkerk A, Meijers JC, Voest EE, Borel RI, Gebbink MF. The role of the fibrinolytic system in corneal angiogenesis. Angiogenesis 2003; 6: 311–6
- Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, et al. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene 2004; 23: 6986–90
- Kwaan HC, Wang J, Svoboda K, Deklerck PJ. Plasminogen activator inhibitor 1 may promote tumour growth by inhibition of apoptosis. Br J Cancer 2000; 82: 1702–8
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000; 57: 25–40