Abstract
The stability of Health-Related Quality of Life (HRQoL) in the general population (GenPop) over years has rarely been evaluated. Neither has the impact of chronic morbidity on HRQoL in cancer survivors been extensively assessed, when identified in the Norwegian GenPop. We studied both aspects. HRQoL was evaluated in two GenPop surveys in 1996 and 2004 using the EORTC QLQ-C30. The 2004 survey included self-reports of a malignant diagnosis and use of medication for hypertension, diabetes mellitus and/or anxiety/depression. Comparison of the results from both surveys revealed similarity of the HRQoL profiles of the two surveys and confirmed the associations between HRQoL and age and gender. Cancer survivors and individuals from the GenPop without chronic co-morbidity had similar HRQoL, except for poorer physical and role function in cancer survivors (p <0.01). HRQoL worsened significantly if a cancer survivor suffered from chronic co-morbidity. Multivariate analyses confirmed the associations between HRQoL and chronic common co-morbidity in cancer survivors and non-cancer persons. As common chronic co-morbidity significantly impairs HRQoL in cancer survivors, prevention of adverse health conditions represents a major challenge in such survivors. Further, in the interpretation of HRQoL in cancer survivors‘ co-morbid conditions and socio-demographic variables must be considered. Over an 8 years period the HRQoL of the Norwegian GenPop appeared to be stable.
During the last decade Health-Related Quality of Life (HRQoL) of the general population (GenPop) has gained increasing interest among clinicians and the public press, and is today considered to be an important outcome variable in oncological research. In Europe The Core Quality of Life Questionnaire of the European Organization for Research and Treatment of Cancer (EORTC QLQ-C30) Citation[1–5], the Short Form 36 (SF-36) Citation[6] or the FACT-G questionnaire Citation[7] are frequently used for assessment of HRQoL in cancer patients and in GenPop. To gain insight into the potential impact of cancer upon HRQoL, reference data are obtained from the GenPop, and have shown that women generally report lower functional status, lower global quality of life, and higher levels of symptoms than men. In addition, functional status tends to decline with increasing age Citation[2–5]. Common chronic non-malignant morbidity (cardiovascular disorders, diabetes, allergy, arthritis/ osteoporosis) reduces HRQoL Citation[8]. Marital and educational status should be taken into account when HRQoL data are interpreted Citation[9], Citation[10].
To the best of our knowledge only one recent study has assessed the stability of HRQoL data from the GenPop over 5 years or more Citation[11]. The authors concluded that HRQoL seemed to be relatively stable across the observation period, however with large inter-individual variations. On the background of changing living conditions over years more studies on stability of reference data from the GenPop appear appropriate.
Few European investigations have been published on population-based HRQoL that compare cancer survivors in general with people with or without common chronic diseases. If such comparisons are done, statistically significant differences in important HRQoL dimensions may not exceed “small changes” Citation[12], thus being of limited clinical relevance. Baker et al. Citation[13] found only slight, though statistically significant differences in the HRQoL dimensions between cancer survivors beyond treatment and a control group. Nord et al. Citation[14] in their population-based survey observed that the odds ratio for reporting “poor health” was only 1.27 in 5 year cancer survivors when compared to GenPop Citation[14]. Self-reported adverse somatic health conditions and health complaints increased these odds ratios to 2.83 and 5.85, respectively. HRQoL data in cancer patients have been obtained most often from investigations, in which health care workers have focused on a specific malignancy. Such studies have shown reduced HRQoL in patients with the cancer type in question compared to GenPop, but they have only rarely assessed the influence of co-existing morbidity in cancer patients as compared to non-cancer individuals. It still remains unclear whether impaired HRQoL in cancer survivors sampled from GenPop is mainly due to the malignant diagnosis per se, or if it can be attributed to co-existing chronic morbidity.
On this background the aim in the present study of Norwegian HRQoL data from GenPop is two-fold. The initial objective was to compare reference data obtained in 2004 by the QLQ-C30 with a sample of the Norwegian GenPop data collected in 1996 Citation[2]. No major changes in HRQoL were expected between the two assessments, as no major changes in living conditions likely to threaten general welfare have taken place in Norway in this time period. We secondarily evaluated the importance of selected chronic adverse health conditions for HRQoL in individuals from the GenPop with or without a cancer diagnosis. We assumed that co-morbidity, rather than the malignant diagnosis itself, would be associated with impaired HRQoL.
Patients and methods
GenPop 2004 cohort
The anonymously performed postal health survey was conducted in 2004 as previously described Citation[15], Citation[16]. An aged-representative sample of 3 500 men and 3 500 women was identified from the general adult Norwegian population with planned over-sampling of the oldest age groups. Those invited were asked to return the completed questionnaire in a pre-stamped envelope. Due to anonymity, no reminder was sent to non-responders.
The questionnaire consisted of a few socio-demographic items (gender, age, education, marital status), the EORTC QLQ-C30 Citation[1], the Fatigue questionnaire (FQ) Citation[17], the Body Image Scale Citation[18], the Brief Sexual Inventory Form (BSIFI) Citation[19] for mens or Sexual Activity Questionnaire (SAQ Citation[20] for womens. Responses to the question “Have you had cancer?” (Yes or No) identified individuals with a cancer diagnosis. Chronic non-malignant morbidity was assessed by a person's use of medication due to hypertension, diabetes mellitus and/or depression/anxiety.
Five groups were constructed: Group 1: Ca + /Mb+: Cancer with co-Morbidity (as defined as any of the above non-malignant disorders): Group 2: Ca + /Mb − ; Cancer without chronic co-Morbidity: Group 3: Ca − /ADe: No Cancer, but anxiety/depression (with or without co-existent hypertension/diabetes); Group 4: Ca − /HDi: No Cancer, but hypertension and/or diabetes mellitus; Group 5: Ca − /Mb−: No Cancer, no co-Morbidity. For comparisons of cancer survivors with somatic and/or psychological co-morbidity and non-cancer individuals with co-morbidity Group 3 and Group 4 were combined into Group 3 + 4(Ca − /Mb + ).
The EORTC QLQ-C30 questionnaire
The present study deals only with HRQoL data from the QLQ-C30 questionnaire. The responses to QLQ-C30 were transformed to scales ranging from 0 to 100 Citation[21]. For inter-group comparisons we selected eight dimensions considered to be of greatest influence on HRQoL. Global Quality of life (GQ), Cognitive function (CF), Emotional function (EF), Physical function (PF), Role function (RF), Social function (SF), Fatigue (FA) and Pain (PA).
For compliant individuals the original data file from the Norwegian 1996 survey was available to the authors Citation[2] for direct comparisons between the results from 1996 and 2004. Comparisons of compliance rates were based on subsequent analysis of the 1996 data Citation[22].
Statistical analyses and methods
The data were analyzed by the statistical software SPSS versions 12 and 13 using standard descriptive statistics. Due to multiple testing, only p-values of <0.01 were considered to be statistically significant, and all tests were two-tailed. Clinically relevant differences between mean scores of the QLQ-C30 dimensions required a difference of ≥10 points Citation[12].
The effect of co-morbidity and socio-demographic variables on selected functioning and symptoms scales of the QLQ- C30 was also assessed by analyses of covariance (ANCOVA). The healthiest population (Group 5) served as the reference category.
Results
Of 7 000 invited individuals, 2 497 returned a completed questionnaire (overall response rate 36%, ). Thirty-nine percent of the women (n = 1 370) and 32% of the men (n = 1 127) responded (p < 0.001). The overall response rate in 2004 was less than half of that in 1996, the difference being particularly large in the two youngest age groups. Compared with the age distributions in the Norwegian population from respectively 1996 and 2004 statistically significant under-representation of the youngest persons became obvious in the 2004 survey with overrepresentation of the two oldest age classes (data not shown).
Table I. Socio-demographic characteristics of the 2004 sample, compared to the 1996 cohort.
Statistically significant differences for demographics were also evident between the 2004 and 1996 survey: The mean age for those who responded in 2004 was 54 years, compared with 46 years in 1996 (p < 0.001). In 2004, 79% of the individuals reported that they were married or had an intimate relationship opposed to 55% of persons who were married in 1996. No significant differences between the two cohorts were observed as to level of education.
Of 2 497 responders, 1 648 (66%) did not report any of the adverse health conditions covered by the questionnaire (). A total of 651 persons without cancer (Group 3 + 4) were on medication for hypertension/diabetes (475) or used anxiolytic or anti-depressive drugs (n = 176). A total of 198 persons (8%) reported that they had a cancer diagnosis, in 84 of them combined with co-morbidity (Group 1) and in 114 without (Group 2). (Due to the limited number of cancer patients with co-morbidity no detailed analyses were done as in cancer patients with hypertension/diabetes (n = 66) as opposed to those with mental distress).
Table II. Socio-demographic characteristics across co-morbidity groups.
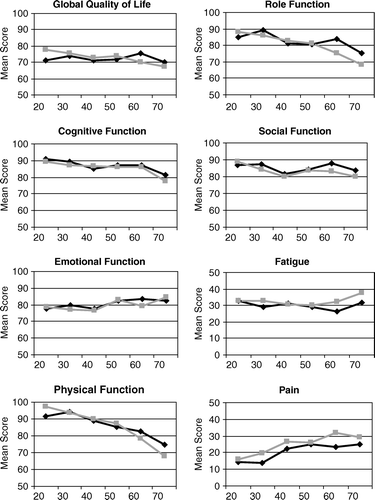
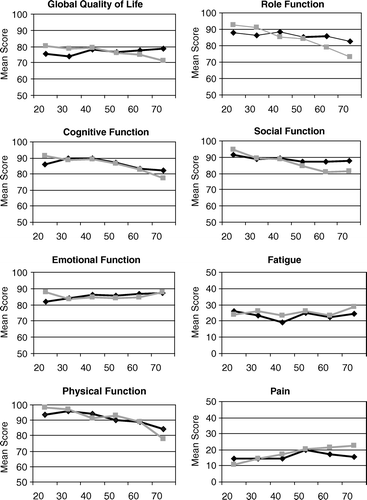
Comparing the age-related means of the HRQoL dimensions from the 2004 cohort with those from the 1996 survey, separately for each gender, no clinically relevant differences emerged () in the 210 comparisons in spite of 14 statistically significant differences (): In 2004 females aged 60 – 69 years reported significantly higher PF scores and lower scores for pain and sleeping problems. On the other hand, the youngest women were characterized by lower physical function and reduced GQ. Reduced means for PF were also typical for the youngest men in the 2004 cohort. GQ did, however, not differ between the total 1996 and the total 2004 cohort. As for the 1996 cohort the gender-related means of the functional dimensions decreased with increasing age, whereas the means of most symptoms scales tended to increase, at least after the age of 30 years ().
Table IIIa. QLQ C-30 in females: (2004 survey); Mean scores and standard deviation.
displays the mean values for selected HRQoL dimensions for the five groups (all age classes, both genders) and for the combined Group 3 + 4. As expected, the highest means of the functional dimensions and lowest means of the symptom scales were observed in Group 5 (Ca − /Mb − ). In individuals without a cancer diagnosis but with hypertension and/or diabetes (Group 4) all means of the functional dimensions were lower and higher for the symptom scores than in Group 5 (Ca − /Mb − ). There were statistically significant differences between these two groups in all dimensions, with clinical relevance only for physical function. Anxiety/depression reduced the mean values for all dimensions by ≥10 points with differences almost up to 30 points between Group 5 and Group 3.
Table IIIb. QLQ C-30 in males (2004 survey): Mean scores and standard deviation.
No clinically relevant differences in any of the examined HRQoL dimensions were observed between Group 5 (Ca − /Mb − ) and Group 2 (Ca + /Mb − ), though the means for PF and RF were reduced in the cancer survivors (p < 0.01). The differences for these latter two dimensions were larger in males (PF: 8 points; RF: 9 points) than in females (PF: 6 points; RF: 5 points) (data not shown).
Cancer patients with co-morbidity (Group 1: Ca + /Mb + ) reported clinically significant reductions of all evaluated HRQoL dimensions compared to Group 5, except for emotional function. Also if compared to Group 2 (Ca + /Mb − ), the scale differences in cancer patients with or without co-morbidity reached the level of clinical relevance for most of the assessed dimensions. However, no statistically significant differences emerged for any of the HRQoL dimensions comparing Group 1 ( Ca + /Mb + ) with Group 3 + 4 (Ca − /Mb + ). The mean GQ score for the 66 cancer patients with hypertension or diabetes mellitus was 65, and was 51 for the 18 cancer patients with anxiety/depression, clinically not different from the comparable means in individuals without cancer but with similar co-morbidity (Data not shown).
In the analyses of covariance performed separately for each of the eight selected HRQoL dimensions as dependent variables and with Group 5 (Ca − /Mb − ) as reference the socio-demographic variables remained independently associated with most of the HRQoL scales (). A cancer diagnosis which was combined with chronic co-morbidity remained significantly associated with reduced GQ and all other HRQoL dimensions, whereas this was the case only for physical and role function in cancer patients without co- morbidity. In individuals without cancer both somatic and mental co-morbidity were associated with significant reduction of all HRQL dimensions.
Table IV. Functioning and symptom scale scores in the 5 sub-groups and in a group combining Group 3 and Group 4.
Table V. Analysis of covariance for functioning and symptom scales in the EORTC QLQ-C30; the effect of comorbidity and sociodemographic characteristics. Unstandardized regression coefficients (B) and 95% Confidence Intervals (95% CI).
Discussion
The present study resulted in two findings of principal clinical interest: over an eight year period, no clinically significant changes in HRQoL were observed in the Norwegian GenPop, as assessed by the EORTC QLQ-C30. Secondly, a cancer diagnosis per se, in an individual without chronic co-morbidity (identified as hypertension, diabetes, anxiety/depression), does not appear to decrease self-reported GQ despite significantly lower mean values for physical and role function. On the other hand, the co-existence of a cancer diagnosis and somatic and/or psychological morbidity decreases the means of all dimensions of the QLQ-C30 significantly compared to both cancer survivors without such co-morbidity and healthy non-cancer persons from GenPop. We also confirmed previously shown strong associations between HRQoL and age, gender, Marital status and education Citation[2–5], Citation[9], Citation[13], both for cancer and non-cancer individuals.
The age-and gender-related HRQoL profiles have remained stable since the first Norwegian survey Citation[2], in spite of the differences in the distribution of age classes in the two surveys. Some of the demographic differences are easily understandable: In 2004 an oversampling of the older age classes was a priori planned as we expected a lower response rate among these individuals. At the same time we were particularly interested in covering the older age classes in which malignant diseases are most common. Obvious differences as to the marital status are possibly explained by inter-survey age differences and slightly different questions used in the surveys: In 1996 the questionnaire only addressed the legalized status of matrimony, whereas the wording in 2004 covered both married and cohabitating couples.
The inter-group comparison resulted in clinically interesting findings: In the absence of common chronic co-morbidity, HRQoL was similar in non-cancer individuals and cancer patients, except for PF and RF. Of particular interest is the fact that HRQoL in cancer survivors was not inferior to that of non-cancer persons with chronic somatic co-morbidity, the means in Group 4 being even above those of Group 2. All HRQoL dimensions were significantly decreased if cancer patients had adverse health conditions; both if compared with persons without cancer and cancer patients without co-morbidity. Psychological distress, in particular has a detrimental effect on HRQoL as demonstrated for Group 3. Alonso et al. showed that arthritis, not assessed in the present study, had the highest impact on the HRQoL of GenPop Citation[8], but mental disorders were not included in their analysis. Our results confirm the observations by Baker et al. Citation[13] as to reduction of self-reported physical function in cancer survivors in general, and that co-morbidity further decreases HRQoL. As in the present study, the survey from the US showed that the absolute differences in the HRQoL dimensions were small comparing cancer survivors with non-cancer persons as long as they were without co-morbidity. Clinically impaired HRQoL became evident first after the co-occurrence of common chronic disorders. Also Garman et al. Citation[23] emphasize the detrimental influence of co-morbidity on the functional status in elderly cancer patients, which is in agreement with the overall findings of Nord et al. Citation[14] of a five-fold increase of “poor health” in cancer patients with health complaints. In addition to common chronic adverse health conditions specific cancer-related symptoms and functional deficits, not covered by the QLQ- C30, may influence on cancer survivors‘ HRQoL, as shown for neuro- and ototoxicity in testicular cancer patients Citation[24] or vasomotor symptoms in breast cancer patients Citation[25], Citation[26]. Our results thus strongly indicate large variations of GQ and other HRQoL dimensions among cancer patients, in relation to co-morbidity. The high means of emotional function in spite of a malignant diagnosis may be explained by “response shift” Citation[27], commonly observed in cancer patients.
Our study has several limitations to be considered: The age distribution in the 2004 cohort is skewed compared to the general population in Norway. Our 2004 sample thus contains too few young and too many older individuals. This is in part due to the pre-determined over-sampling of the oldest age groups. Further, 8% of the responders were cancer survivors, a percentage which is more than twice the prevalence of cancer survivors in the Norwegian population (approximately 3.5%). The comparable figure in the Medicare-based survey of Baker et al. Citation[13] was 14%. It is difficult to estimate how this over-representation of cancer survivors has influenced on the final results.
The overall response rate in our study was only 36%. Our low response rate in the 2004 cohort may be associated with the length of the mailed questionnaire, which contained four validated instruments. In particular, the questions on sexuality may have increased the non-response rate, as also discussed previously Citation[16]. Furthermore, during the last years GenPop in Norway has increasingly been asked to participate in surveys and this might have resulted in an overall “tiredness” to respond to our mail. Finally, contrary to the data collection procedure in 1996, no reminder was sent to non-responding individuals.
Similar to the report by Baker et al. Citation[13] we did not ask for year of cancer diagnosis or the cancer type. We also lack information on current disease activity and treatment. HRQoL in cancer survivors is certainly dependent on the time since diagnosis, the type of malignancy, the presence or absence of disease manifestations and whether treatment was ongoing or had been discontinued. The lack of these medical parameters should be viewed on the background of the study design: Primarily we planned to assess stability over time as the only aim of the 2004 survey, and we were aware of the fact that the 1996 survey did not contain any questions about chronic adverse health conditions. The second objective of the present study emerged during the analysis of the data collected in 2004.
Finally, we restricted our definition of co-morbidity to only three diagnoses. Other co-morbidities are frequently prevalent in cancer patients and in non-cancer persons as arthritis/ osteoporosis and cardiac diseases Citation[14]29 and should be included in future surveys.
The strength of our study is the relatively large number of patients and the population-based design of the study, both providing more background for understanding the term “Cancer survivorship”.
Conclusion
In spite of the limitations emerging from different sampling procedures in 1996 and 2004, the HRQol of the Norwegian population as measured by the QLQ-C30 has remained relatively stable over a period of 8 years. A malignant diagnosis is per se not associated with reduced GQ, though cancer reduces physical and role function. Significant impairment of HRQoL emerges if cancer patients develop common co-morbidities (hypertension, diabetes mellitus and/or psychological distress). Our results therefore challenge oncologists and family doctors in their tasks to prevent common somatic co-morbidity and to treat existing anxiety/depressive disorders to sustain optimal HRQoL in cancer survivors. Further, the term “cancer survivor” implies great heterogeneity, emphasizing the need to always assess a minimum set of socio-demographic and medical variables (gender, age, co-morbidity) in addition to the specific study parameters in follow-up studies.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ = C30 (+3). J Clin Oncol 1998; 16: 1188–96
- Michelson H, Bolund C, Nilsson B, Brandberg Y. Health-related quality of life measured by the EORTC QLQ-C30. Acta Oncol 2000; 39: 477–84
- Persson LO, Karlsson J, Bengtsson C, Steen B, Sullivan M. The Swedish SF-36 Health Survey II. Evaluation of clinical validity: Results from population studies of elderly and women in Gothenborg. J Clin Epidemiol 1998; 51: 1095–103
- Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer 2001; 37: 1345–51
- Ware JE, Jr, Kosinski M, Gandek B, Aaronsen NK, Apolone G, Bech P, et al. The factor structure of the SF-36 Health Survey in 10 countries: Results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51: 1159–65
- Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof 2005; 28: 192–211
- Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: Results from the International Quality of Life Assessment (IQOLA) Project. Quality of Life Res 2004; 13: 283–98
- Loge JH, Foss Abrahamsen A, Ekeberg Ø, Kaasa S. Reduced health-related quality of life among Hodgkin‘s disease survivors: A comparative study with general population norms. Ann Oncol 1999; 10: 71–7
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on Quality of life – the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3). Eur J Cancer 1998; 34: 1381–9
- Hopman WM, Berger C, Joseph L, Towheed T, VandenKerkhof E, Anastassiades T, et al. The natural progression of health-related quality of life: Results of a five-year prospective study of SF-36 scores in a normative population. Qual Life Res 2006; 15: 527–36
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–44
- Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare Managed Care. Cancer 2003; 97: 674–81
- Nord C, Mykletun A, Thorsen L, Bjoro T, Fossa SD. Self-reported health and use of health care services in long-term cancer survivors. Int J Cancer 2005; 114: 307–16
- Mykletun A, Dahl AA, O'Leary MP, Fossa SD. Assessment of male sexual function by the Brief Sexual Function Inventory. Br J Urol Int 2006; 97: 316–23
- Vistad, I, Fosså, SD, Kristensen, GB, Mykletun, A, Dahl, AA. The sexual activity Questionnaire –psychometric properties and normative data in a Norwegian population sample. J. Women's Health in press (2007).
- Chalder T, Berelowitz G, Pawilowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J psychosom Res 1993; 37(2)147–53
- Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer 2001; 37: 189–97
- O'Leary MP, Fowler FJ, Lenderking WR, et al. A brief male sexual function inventory for urology. Urology 1995; 46: 697–706
- Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: A measure of women's sexual functioning. Qual Life Res 1996; 5: 81–90
- Fayers P, Aaronson NK, Bjordal K, Sullivan M. EORTC QLQ C-30 scoring manual. EORTC publications, Brussels 1997
- Kaasa S, Hjermstad MJ, Jordhøy MS, Wisloff F, Loge JH. Compliance in quality of life data: A Norwegian experience. Stat Med 1998; 17: 623–32
- Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci 2003; 58: 1119–24
- Mykletun A, Dahl AA, Haaland CF, Bremnes R, Dahl O, Klepp O, et al. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J Clin Oncol 2005; 23: 3061–8
- Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs 2001; 17: 241–8
- Ganz PA. Breast cancer, menopause, and long-term survivorship: Critical issues for the 21st century. Am J Med 2005; 118: 1365–415
- Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol 1999; 10: 747–9
- Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Health profiles in 5836 long-term cancer survivors. Int J Cancer 2003; 104: 488–95