Abstract
The use of untraditional treatment modalities for external beam radiotherapy such as intensity modulated radiation therapy (IMRT) and proton beam therapy is increasing. This review focuses on the changes in the dose distribution and the impact on radiation related risks for long-term cancer survivors. We compare conventional radiotherapy, IMRT, and proton beam therapy based on published treatment planning studies as well as published measurements and Monte Carlo simulations of out-of-field dose distributions. Physical dose parameters describing the dose distribution in the target volume, the conformity index, the dose distribution in organs at risk, and the dose distribution in non-target tissue, respectively, are extracted from the treatment planning studies. Measured out-of-field dose distributions are presented as the dose equivalent as a function of distance from the treatment field. Data in the literature clearly shows that, compared with conventional radiotherapy, IMRT improves the dose distribution in the target volume, which may increase the probability of tumor control. IMRT also seems to increase the out-of-field dose distribution, as well as the irradiated non-target volume, although the data is not consistent, leading to a potentially increased risk of radiation induced secondary malignancies, while decreasing the dose to normal tissues close to the target volume, reducing the normal tissue complication probability. Protons show no or only minor advantage on the dose distribution in the target volume and the conformity index compared to IMRT. However, the data consistently shows that proton beam therapy substantially decreases the OAR average dose compared to the other two techniques. It is also clear that protons provide an improved dose distribution in non-target tissues compared to conventional radiotherapy and IMRT. IMRT and proton beam therapy may significantly improve tumor control for cancer patients and quality of life for long-term cancer survivors.
External beam radiotherapy technology has gone through a period of rapid developments that are now being implemented on a large scale in clinical practice. The main rationale is increased conformality of treatment delivery, which can result in lower doses to surrounding normal tissues, allowing possible dose escalation to the tumor for the same normal tissue toxicity. The aim of this paper is to show how the introduction of untraditional treatment techniques, such as intensity modulated radiation therapy (IMRT) and proton beam therapy, influences external beam radiotherapy dose distributions. The impact for long-term survivors with risk for late effects, secondary malignances as well as on tumor control is also discussed. We compare different treatment modalities; conventional radiotherapy (this includes standard ‘non-conformal’ technique, and 3D conformal radiotherapy (3DCRT)), IMRT (step and shoot, dynamic MLC, serial and helical tomotherapy), and proton beam therapy (passive and active scattering), by presenting a review of published data. Since clinical data are scarce, treatment planning studies and dose distribution studies are the basis of this review.
Three dimensional imaging is fundamental for modern radiotherapy treatment planning. Imaging techniques introduced in the last decade, such as MRI, PET-CT etc, improve the definition of volumes of interest in the patient, i.e. tumor, positive nodes, and organs at risk (OARs). In addition, image-guided radiotherapy and respiratory gating will allow more precise treatment delivery. These improvements will be of great importance for the long-term survivors after radiation treatment, but will not be covered here.
The number of fields are often few in standard photon beam radiotherapy. However, in 3DCRT, and particularly in IMRT, a large number of fields are used in order to improve the conformality of the dose distribution to the target volume. The high doses present in the irradiated non-target volume in standard radiotherapy are then spread out over a larger volume, resulting in low to medium dose levels surrounding the target volume. In proton beam radiotherapy the reverse is often the case, with few beams and a fast dose fall-off behind the target volume. The steep fall-off is the result of the Bragg peak, which is located in the target volume. The dose at the exit side of the target is negligible. For photon beams, the absorbed dose to out-of-field normal tissue (the peripheral dose) is mainly due to photon scatter originating in the irradiated volume and leakage from the treatment unit. Peripheral dose due to photons is a function of several factors, including distance from the field edge and field size Citation[1–3]. Scatter dominates close to the treatment field, while leakage is more isotropic and therefore varies less with distance, and dominates away from the field. The leakage dose is proportional to the beam-on time, and is therefore higher for IMRT than for conventional radiotherapy. For linacs operating above ∼10 MV there is an additional contribution from neutrons generated by (γ,n) reactions in the treatment head. The increased beam-on time results in increased secondary neutron production for high-energy IMRT, which can contribute significantly to the equivalent dose, as neutrons have a high relative biological effect. Neutrons are also an undesirable contaminant source of radiation in proton beam therapy.
Comparison of treatment planning studies
The important question remains whether or not the use of new technologies, such as IMRT or proton beams, will considerably improve the clinical outcome for the patient. Long-term clinical experience using these techniques is still rare. A very limited number of randomized clinical trials have been performed, studying the effect of introducing new technology. Some patient data exist, however, that indicate that more recent techniques have improved the clinical outcome Citation[4–6]. In the absence of clinical evidence, treatment outcome is simulated here by treatment planning studies. A large number of treatment planning studies have been published in the last years (see listing in reference Citation[7]). The requirements for selection of publications in this review are that they contain IMRT plans, and at least some CT planned patients. Investigations with several patients and with the same disease are primarily included, see . Apart from IMRT, the selected publications include conventional external beam radiotherapy, and/or proton beam therapy. The treatment modalities are grouped into these three main techniques, as very few investigations have used exactly the same techniques. Other differences among the studies include the beam arrangement, the treatment planning system, optimization algorithm, etc. These variations are likely to influence the dose distribution and thus the comparison, which adds to the uncertainty of the result.
Table I. List of treatment planning studies evaluated in this review.
We compare the dose distribution in three different volumes; the target volume, organs at risk, and non-target tissue. Treatment planning comparisons are mainly evaluated using physical dose parameters. Some authors also apply dose response models for probability of tumor control (TCP), as well as probability of normal tissue complications (NTCP) Citation[8], Citation[9]. The equivalent uniform dose (EUD) is also used Citation[10]. Unfortunately, the presentation of the dose distributions obtained in the different investigations is very heterogeneous, using a number of parameters, which complicates comparisons. A single common dose parameter per volume does not exist. Therefore one or mean values of a specific representative dose parameter per volume, per technique and per investigation are selected (). In the OARs, two dose parameters are chosen representing maximum and average dose, respectively. Dose parameters for conventional radiotherapy and proton beam therapy are presented as percentage units relative to IMRT. A negative value indicates that the technique is inferior to IMRT, a positive value indicates that the technique is superior to IMRT.
Table II. The different dose parameters used in order to describe the dose distribution in the different volumes of interest: D95 (dose in percent where the cumulative DVHs intersect with 95% volume), V30, V50, V70, V95 (volume in percent where the cumulative DVHs intersect with 30, 50, 70 95% dose level, respectively), coverage ([target dose ≥ desired dose]/PTV), minimum significant dose (measure of the magnitude of the cold spot of a significant volume), inhomogeneity coefficient ([D5 − D95]/D95), integral dose (mean dose times volume irradiated to any dose), maximum significant dose (measure of the magnitude of the hot spot of a significant volume).
Dose distribution in target volume
shows the superior/inferior relation to IMRT of the dose distribution in the target volume. For head and neck (H&N) tumors the dose distribution improves using IMRT compared to conventional radiotherapy. There seems to be a further small improvement using proton beams. Similar improvements are also demonstrated for breast. The irregular and sometimes complex shape of the target volume in H&N and breast, and all OARs in the surrounding are probably the reasons for the improvements. For target volumes located in or abutting the spinal cord Citation[11], Citation[12] the changes in the dose distribution between the three techniques show large variations and no general conclusion can be drawn due to the limited number of data. In the pelvic region the data also varies and no clear difference in the dose distribution between the three techniques is seen. The shape of the target volume and surrounding OARs is often less complex than in H&N region. This might be the reason why no clear difference between the three techniques can be seen in pelvic region.
Figure 1. Dose distribution in target volume. Data from treatment planning studies Citation[8–12], Citation[49–56], see for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: D95 (dose in percent where the cumulative DVHs intersect with 95% volume), V95 (volume in percent where the cumulative DVHs intersect with 95% dose level), coverage ([target dose ≥desired dose]/PTV), minimum significant dose (measure of the magnitude of the cold spot of a significant volume), inhomogeneity coefficient ([D5–D95]/D95). Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.
![Figure 1. Dose distribution in target volume. Data from treatment planning studies Citation[8–12], Citation[49–56], see Table I for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: D95 (dose in percent where the cumulative DVHs intersect with 95% volume), V95 (volume in percent where the cumulative DVHs intersect with 95% dose level), coverage ([target dose ≥desired dose]/PTV), minimum significant dose (measure of the magnitude of the cold spot of a significant volume), inhomogeneity coefficient ([D5–D95]/D95). Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.](/cms/asset/d61ef4e0-d790-4306-a4fc-62eb4317b2fe/ionc_a_221782_f0001_b.jpg)
Some of the investigations Citation[8], Citation[9] have calculated the TCP. For hypopharyngeal tumor Johansson et al. Citation[9] demonstrate significant improvements of TCP for both IMRT (54%) and proton beam therapy (55%) compared to conventional radiotherapy (38%). For prostate tumors the TCP was very similar for all techniques; conventional radiotherapy (93%), IMRT (93%), and proton beam therapy (95%). The results obtained with TCP support the results obtained using dose parameters in H&N and pelvic regions. In the H&N region a significant improvement of target dose distribution can be expected using untraditional treatment techniques such as IMRT or proton beams compared to conventional techniques. On the other hand the three techniques show almost similar target dose distribution and TCP values in pelvic region.
Conformity index
shows the superior/inferior relation to IMRT of the conformity index (CI). For all treatment sites the CI of the dose distribution around target is not substantially improved when proton beams are compared to IMRT, but, as expected, the conventional technique has a much lower CI regardless of treatment site. Noteworthy is that none of the three modalities achieve an ideal CI = 1, which indicates a perfect match of the treated volume to the target volume.
Figure 2. Conformity index. Data from treatment planning studies Citation[10], Citation[12], Citation[50], Citation[54–56], see for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The conformity index is based on the 90% or the 95% isodose line. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.
![Figure 2. Conformity index. Data from treatment planning studies Citation[10], Citation[12], Citation[50], Citation[54–56], see Table I for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The conformity index is based on the 90% or the 95% isodose line. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.](/cms/asset/615ce21f-0192-4146-91b5-652b3f397534/ionc_a_221782_f0002_b.jpg)
Dose distribution in OAR
shows the superior/inferior relation to IMRT of the maximum dose in the different OARs located in the vicinity to or abutting the target volume. The maximum dose in the different OARs for conventional technique is much higher than for the other techniques. For proton beams the data varies to a large extent and no general conclusion can be drawn. The size of the penumbra greatly influences the maximum dose to OARs that are directly abutting the target volume. Therefore, the size of the penumbra has a large impact on CI and on the maximum dose to OAR volumes that are adjacent to the target volume. For paraspinal sarcomas, comparing IMRT and proton beams, Weber et al. Citation[12] conclude that maximum dose to spinal cord as well as the dose distribution in the target and CI are similar.
Figure 3. OAR maximum dose. Data from treatment planning studies Citation[8], Citation[12], Citation[50], Citation[52], Citation[53], Citation[55], see for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: maximum dose, maximum significant dose (measure of the magnitude of the hot spot of a significant volume), V95 (volume in percent where the cumulative DVHs intersect with 95% dose level. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering. Note that values for refs Citation[8], Citation[50] extend beyond the scale.
![Figure 3. OAR maximum dose. Data from treatment planning studies Citation[8], Citation[12], Citation[50], Citation[52], Citation[53], Citation[55], see Table I for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: maximum dose, maximum significant dose (measure of the magnitude of the hot spot of a significant volume), V95 (volume in percent where the cumulative DVHs intersect with 95% dose level. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering. Note that values for refs Citation[8], Citation[50] extend beyond the scale.](/cms/asset/a50ab22d-569e-4d0f-964e-a264127d8962/ionc_a_221782_f0003_b.jpg)
There is a large improvement, as expected, in the average dose to the OAR for proton beams compared to IMRT (). The data consistently shows that proton beam therapy substantially decreases the OAR average dose compared to the other two techniques. However, no systematic difference is demonstrated between IMRT and conventional technique for this parameter.
Figure 4. OAR average dose. Data from treatment planning studies Citation[9–12], Citation[49–55], see for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: mean dose, V30, V50 (volume in percent where the cumulative DVHs intersect with 30 and 50% dose levels, respectively), dose to 2/3 volume. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.
![Figure 4. OAR average dose. Data from treatment planning studies Citation[9–12], Citation[49–55], see Table I for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: mean dose, V30, V50 (volume in percent where the cumulative DVHs intersect with 30 and 50% dose levels, respectively), dose to 2/3 volume. Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.](/cms/asset/ea3872a8-0372-48e8-b57f-6453934ae52b/ionc_a_221782_f0004_b.jpg)
Some of the investigations Citation[8], Citation[9] have estimated the NTCP. For hypopharyngeal tumor Johansson et al. Citation[9] demonstrate significant improvements of NTCP for parotid glands for both IMRT (58%) and proton beams (41%) compared to conventional radiotherapy (93%). The mainly parallel structure and dose-volume dependence of parotid is the reason for the lower NTCP with proton beams relative to IMRT, since the average dose in parotid is lower with proton beams. For rectum the estimated NTCP was significantly higher with conventionl technique (16%) compared to the other techniques (4–8%) Citation[8]. The results estimated from the NTCP model show good correlation to the maximum rectal dose, and to average dose in the parotid.
Dose distribution in non-target tissue
For IMRT, the intensity optimization procedure can effectively remove high doses from critical structures and redistribute this dose elsewhere within the body outline. Treatment planning studies disagree on the effect of using IMRT compared to conventional radiotherapy on the average dose to non-target tissue ().
Figure 5. Non-target tissue average dose. Data from treatment planning studies Citation[8–11], Citation[49–56], see for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: mean dose, V30, V50, V70 (volume in percent where the cumulative DVHs intersect with 30, 50 and 70% dose level respectively), integral dose (mean dose times volume irradiated to any dose). Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.
![Figure 5. Non-target tissue average dose. Data from treatment planning studies Citation[8–11], Citation[49–56], see Table I for an overview. First author, reference number, treatment site, and value are shown. A positive value indicates that the technique is superior to IMRT. The following physical dose parameters are included: mean dose, V30, V50, V70 (volume in percent where the cumulative DVHs intersect with 30, 50 and 70% dose level respectively), integral dose (mean dose times volume irradiated to any dose). Conventional radiotherapy includes standard non-conformal technique and 3DCRT; IMRT includes step and shoot, dMLC, serial and helical tomotherapy; proton beam therapy includes passive and active scattering.](/cms/asset/f17f2740-dcb1-4b3d-9778-8947a2e3a82c/ionc_a_221782_f0005_b.jpg)
Numerous publications predict that the use of protons provide an improved dose distribution in non-target tissues compared to conventional radiotherapy and IMRT. According to the reduction in average dose to non-target tissue for proton treatment plans compared to IMRT is smaller in the H&N region than in the pelvic region. Planning target volume (PTV) relative to the volume outline of the patient is important for the average dose to the non-target tissue. In the H&N region this volume ratio is often quite large, since the PTV often occupies a significant part of the outline. However, in the pelvic region this ratio is small, i.e. prostate PTV is much smaller than the body outline. In a region with a large amount of non-target tissue compared to the PTV, e.g. prostate tumors, the finite and energy dependent proton range effectively reduces the dose to tissues behind the Bragg peak. However the lateral penumbra of proton beams is of similar size as for a 6 MV photon beam. One drawback of proton beams compared to the other techniques is the limited skin sparing effect. This characteristic can be problematic in some clinical situations, particularly for superficial tumors, resulting in severe skin reaction.
Out-of-field dose distribution
Measured out-of-field dose distributions are often presented as the dose equivalent as a function of distance from the treatment field. We present comparisons of dose equivalent vs. distance, given as the fraction of the treatment dose, as well as effective dose and estimated ‘whole-body’ dose equivalent, for photon and proton beams. Both measured and Monte Carlo (MC) calculated data are considered. 6 MV and 18 MV photon beams are shown separately, due to the neutron component that is only present for the higher energy. Early work on conventional radiotherapy are not all shown, they are represented by Van Der Giessen et al. Citation[13] and Stovall et al. Citation[3], Citation[14].
show the dose equivalent outside the treatment field as a function of distance from the field edge for 6 MV photons, 18 MV photons, and protons, respectively. They will be discussed below. To correct for different dose normalization, data originally presented as percentage of the central axis maximum dose are recalculated to dose equivalent per central axis dose at 5 cm and 10 cm depth, for 6 MV and 18 MV, respectively, by applying standard depth dose or TMR values as published in Supplement No. 25 of the British Journal of Radiology Citation[15].
Figure 6. Dose equivalent per treatment gray as a function of the distance from the field edge. Data for 6 MV photon beams, (approx.) 10×10 cm field size at 10 cm depth is shown unless legend states otherwise. Legend states first author, delivery technique, and reference number. IMRT_m: IMRT based on MLC, ST: serial tomotherapy, HT: helical tomotherapy. Data originally presented as percentage of the central axis maximum dose are recalculated to dose equivalent per central axis dose at 5 cm depth by applying standard depth dose or TMR values Citation[15]. Reference Citation[1] recalculated assuming 340 MU/Gy Citation[40].
![Figure 6. Dose equivalent per treatment gray as a function of the distance from the field edge. Data for 6 MV photon beams, (approx.) 10×10 cm field size at 10 cm depth is shown unless legend states otherwise. Legend states first author, delivery technique, and reference number. IMRT_m: IMRT based on MLC, ST: serial tomotherapy, HT: helical tomotherapy. Data originally presented as percentage of the central axis maximum dose are recalculated to dose equivalent per central axis dose at 5 cm depth by applying standard depth dose or TMR values Citation[15]. Reference Citation[1] recalculated assuming 340 MU/Gy Citation[40].](/cms/asset/b3279966-aec9-416d-ac14-5b994a9d1f84/ionc_a_221782_f0006_b.jpg)
Figure 7. Dose (equivalent) per treatment gray as a function of the distance from the field edge for 18 MV photons. Legend states first author, delivery technique, and reference number. IMRT_m: IMRT based on MLC. Data originally presented as percentage of the central axis maximum dose are recalculated to dose equivalent per central axis dose at 10 cm depth by applying standard depth dose or TMR values Citation[15]. Reference Citation[1] recalculated assuming 150 MU/Gy and 280 MU/Gy for conventional radiotherapy and IMRT, respectively Citation[40]. Right hand scale to be used for references Citation[14], Citation[18], as the neutron component is not included.
![Figure 7. Dose (equivalent) per treatment gray as a function of the distance from the field edge for 18 MV photons. Legend states first author, delivery technique, and reference number. IMRT_m: IMRT based on MLC. Data originally presented as percentage of the central axis maximum dose are recalculated to dose equivalent per central axis dose at 10 cm depth by applying standard depth dose or TMR values Citation[15]. Reference Citation[1] recalculated assuming 150 MU/Gy and 280 MU/Gy for conventional radiotherapy and IMRT, respectively Citation[40]. Right hand scale to be used for references Citation[14], Citation[18], as the neutron component is not included.](/cms/asset/4c338999-619b-4346-9100-7c51511b205b/ionc_a_221782_f0007_b.jpg)
Figure 8. Neutron dose equivalent per proton treatment gray as a function of the distance from the proton field edge. Legend states first author, reference number, proton beam energy, and spread-out Bragg peak.
6 MV photons
compares conventional radiotherapy, IMRT using the MLC, serial and helical tomotherapy, gamma knife, and CyberKnife. Data for 6 MV photons is shown unless stated otherwise. Ion chambers and TLDs were used in most studies.
The data for conventional fields are based on measurements in phantom irradiated by a single field; if available, we present the results for 10×10 cm field size at 10 cm depth. Van Der Giessen Citation[13] estimated the peripheral dose for energies 4–25 MV based on measured data from several publications. This data is in good agreement with the data for 6 MV from Epstein et al. Citation[16], measured on accelerators without multileaf collimator (MLC). Mutic et al. Citation[17], Citation[18] show that the MLC provides additional shielding which reduces the peripheral dose. Stern et al. Citation[19] present values measured on an accelerator equipped with an MLC that are higher than Mutic's. This might be explained by the measurement technique used by Stern et al., as diodes are likely to over-respond outside the field where the photon energy is lower than in the center of the field. The data by Mazonakis et al. Citation[20] is for a 13.5×17.5 cm field, and is in good agreement with data for a 14×14 cm field Citation[21].
For IMRT using the MLC, two clinical cases are shown; a prostate case with eight fields Citation[1], and a small volume H&N case with seven fields Citation[22]. Points close to the field have values similar to Mutic's data for conventional fields. Further away the peripheral dose is higher for IMRT, higher for the prostate case than for the H&N case. This is expected, as the treatment volume is larger for the prostate case than for the H&N case.
The peripheral dose for a static 5×40 cm helical tomotherapy field is low; Monte Carlo calculated results from Jeraj et al. Citation[23] are in good agreement with measured data from Ramsey et al. Citation[24] for the points shown. However, Ramsey et al. show that the peripheral dose is considerably larger for a clinical case. Compared with helical tomotherapy, two studies on the peripheral dose from serial tomotherapy of H&N tumors show considerably higher values Citation[25], Citation[26], especially the data presented by Meeks et al. Citation[25]. As opposed to the other presented measured data, the data by Meeks et al. is measured in-vivo, using optically stimulated luminescence dosimeters typically used for personnel dosimetry placed on nine patients during treatment. Also, the beam energy is higher, 10 MV. The highest peripheral doses that we found in the literature for 6 MV photons are measured for the CyberKnife Citation[22]. Petti et al. Citation[22] assign the large values to leakage radiation. A gamma knife treatment (60Co) results in considerably lower values than the CyberKnife for similar conditions.
The peripheral dose is shown to decrease with increasing distance, and to increase with field size/target volume. It is higher for IMRT than for conventional radiotherapy, especially further away from the field. This is expected as increased beam-on time leads to increased leakage dose, which is of greater relevance further away from the field; while scatter in the treatment volume, which is the main component of peripheral dose close to the field edge, is less affected. At large distances (>30 cm) the factor by which the peripheral dose for dynamic IMRT fields increase compared with static fields is roughly the same as the increase in monitor units (MU) Citation[21]. For dynamic IMRT, assuming that the degree of modulation is similar resulting in similar average gap widths, the increase in peripheral dose with field size is not only due to a larger contribution of scatter from the target volume, but also due to a larger relative increase in MU. This is represented in by Sharma et al. Citation[21], for a 6×6 cm and a 14×14 cm field delivered with slit fields of 2 cm width. The same authors also show a considerable increase in peripheral dose as the degree of modulation increases, represented by smaller slit field widths (data not shown).
18 MV photons
A comparison of published data Citation[1], Citation[14], Citation[18], Citation[27], Citation[28] on peripheral dose as a function of the distance from an 18 MV photon field is shown in . Data for conventional radiotherapy and IMRT are presented. For studies that measured the neutron and photon contributions separately, the total is shown here. The data for a 10×10 field from Stovall et al. Citation[14] and for a 10×10 MLC field by Mutic Citation[18] is due to photons only. As expected, values that include both the photon and the neutron component are higher than data that exclude the neutron component. The peripheral dose is shown to decrease with increasing distance, and to increase with field size. The dose equivalent is higher for IMRT than for conventional radiotherapy, especially at larger distances from the field.
Proton beam therapy
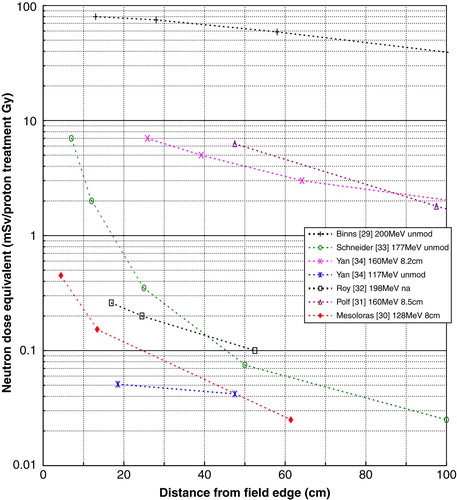
A comparison of published data Citation[29–34] on neutron dose equivalent per proton treatment gray as a function of the distance from the proton field edge is shown in . Both beam line designs (spot scanning and passive scattering) are represented, the energy range is 117 to 200 MeV, and the range modulation varies from unmodulated up to an 8.5 cm spread-out Bragg peak (SOBP). The data series with the highest values was measured during commissioning of a new facility Citation[29]. Additional shielding was introduced, which reduced the dose equivalent with a factor of 100 Citation[35]. The data series with the lowest values was measured for an unmodulated radiosurgery beam, with proton energy 117 MeV Citation[34].
The dose outside a proton treatment field due to secondary neutrons decreases with increasing distance, and with decreasing beam energy. The peripheral dose also depends on the range modulation; measurements by Yan et al. Citation[34] show an increase in neutron dose equivalent by a factor of 10–20 for an 8.2 cm SOBP compared to an unmodulated beam; the neutron dose equivalent increased by a factor of 3–4 with the introduction of 15 cm range modulation, according to MC simulations by Polf and Newhauser Citation[31]. The geometry and materials of the beam delivery system also influences the peripheral dose. This explains some of the large spread of data in ; the spread is also due to different measurement techniques, the use of different neutron quality factors, and large uncertainties of both measurements and MC simulations.
The spot scanning method is expected to have a much lower neutron background than the passive scattering method Citation[33], Citation[36]. Recent measurements for passive scattering units Citation[30], Citation[32] are comparable to or even lower than the data presented for spot scanning. Due to the many different factors that affect the neutron production and the derived dose distribution no conclusion can be drawn from the published data as to which delivery method is preferable regarding neutron background.
Effective dose and ‘whole-body’ dose equivalent
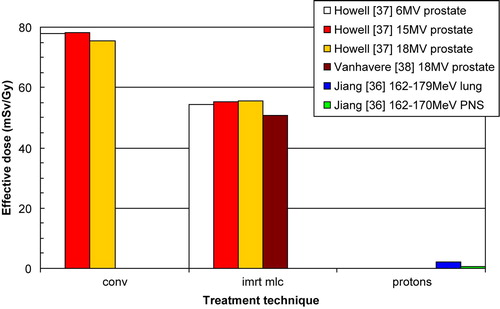
In order to perform an assessment of risk due to various radiotherapy modalities several authors have determined values of effective dose and whole-body dose equivalent. We found three publications that estimate the effective dose (E) by calculating a weighted average of the equivalent dose (HT) to different body tissues (). The equivalent doses to various organs were determined from measurements Citation[37], Citation[38], or Monte Carlo simulation Citation[36], and tissue weighing factors from ICRP-60 (1990) were used to calculate the effective dose. Howell et al. Citation[37] show that the effective dose for IMRT of the prostate is lower compared with conventional radiotherapy for 6 MV, 15 MV, and 18 MV. The investigators attribute this to the fact that organs far from the treatment volume receives higher equivalent doses from IMRT, but at the same time IMRT greatly reduces the dose to nearby organs, such as the bladder and colon, yielding lower effective dose than conventional radiotherapy. The contribution to the effective dose from the bladder and colon is approximately 70%. The total effective dose determined by Vanhavere et al. Citation[38], measured for a prostate treatment in a Rando-Alderson phantom, is in good agreement with the value presented by Howell et al. Citation[37] for 18 MV IMRT. Jiang et al. Citation[36] present data for proton beam treatments of the lung and paranasal sinus. The primary dose distribution is not included, only dose due to secondary neutrons, which is likely to considerably underestimate the effective dose as can be seen below Citation[33].
Figure 9. Effective dose per unit gray delivered to isocenter. Legend states first author, reference number, beam energy, and treatment site. The proton data includes dose due to secondary neutrons only.
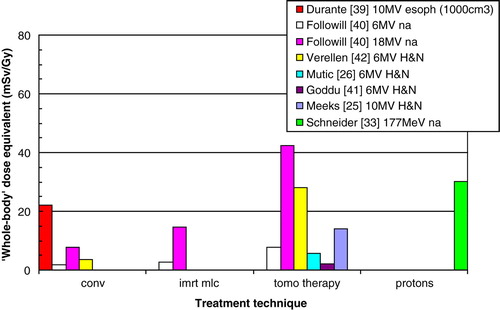
Other risk estimates are based on an estimate of the ‘whole-body’ dose equivalent taken as the dose equivalent delivered to one point, usually 50 cm from the center of the treatment field () Citation[25], Citation[26], Citation[33], Citation[39–42]. Followill et al. Citation[40], Mutic et al. Citation[26], and Goddu et al. Citation[41] estimate the photon and neutron whole body dose equivalent by measurements in phantom. Verellen and Vanhavere Citation[42], and Meeks et al. Citation[25] base their estimates on in-vivo measurements using detectors designed for personnel monitoring. The value for protons Citation[33] is derived by measurements and MC and includes dose deposited by protons as well as neutrons; the dose due to secondary neutrons is small compared to the primary radiation (about 10% or lower). Durante Citation[39] uses cytogenetic methods, which are not based on a single point. Based on the ‘whole-body’ dose equivalent concept, the risk for radiation induced malignancies, assumed to be proportional to the ‘whole-body’ dose equivalent, increases by a factor of 8 for IMRT compared with conventional techniques Citation[40], Citation[42]. This conclusion is quite contrary to Howell's, and is a result of that the ‘whole-body’ dose estimate does not take into account the dose distribution close to the target volume.
Discussion and conclusions
This review clearly demonstrate that a more favorable dose distribution in OARs and non-target tissue can be achieved using proton beam therapy compared to IMRT, which may reduce the risk for normal tissue complications and secondary malignancies. IMRT and proton beam therapy have a similar ability to improve the dose distribution in the target volume (which may increase the probability of tumor control) as well as the dose conformality compared to conventional radiotherapy presently used as standard treatments at most centers world-wide, and also to reduce the maximum dose to OARs. No significant difference is seen between these two techniques in this regard. This is expected, since the lateral penumbra for proton beams and 6 MV photon beams are almost equal, somewhat depending on the depth Citation[12]. This means that for OARs mainly of serial structure as well as all small OARs abutting the PTV, the risk for complications will be similar for IMRT and proton beams, and less than for conventional radiotherapy. For large OARs and OARs of mainly parallel structure proton beam therapy can offer significant tissue sparing, likely preserving the organ function or at least significantly reducing the risk for damage. One caveat of proton beams is that the skin sparing effect is reduced Citation[12]. Weber et al. Citation[12] show that the median skin doses are roughly a factor of 3 better with IMRT than with proton beams.
For the treatment planning studies mostly relative differences between conventional photon beams and IMRT, and proton beams and IMRT, are presented here. Ideally, absolute differences should be reported Citation[43], however this was not possible due to limitations in the data. Also, we chose to present values for effective dose instead of risk for radiation induced secondary cancer, to avoid additional uncertainties due to theoretical models.
Knowledge on late effects is based on experiences from conventional radiotherapy. As mentioned, estimations of risk for radiation induced secondary malignancies are uncertain. Therefore patient studies are preferable. Based on patient studies, secondary tumor induction is shown to be relatively rare but significant in conventional radiotherapy Citation[44–46]. Hall and Wuu Citation[47] estimated that IMRT would increase the incidence of second malignancies by a factor of two compared to conventional radiotherapy. For conventional radiotherapy of prostate cancer, the estimated risk of developing a radiation-associated second malignancy is 1 in 290 for all patients, and 1 in 70 for long-term survivors (≥10 years) Citation[46]. These results are based on a large number (>120 000) of prostate patients, who received either conventional radiotherapy or surgery. For IMRT, this corresponds to an estimated risk of 1 in 145 for all patients, based on Hall and Wuu. Recent results from the largest study so far of 561 men with prostate cancer treated with 15 MV IMRT (dMLC) show that, to date, none of the men developed secondary cancers as a result of the radiation therapy, after a median follow-up period of seven years Citation[48]. This review shows no consistent dose difference between MLC based IMRT and conventional radiotherapy in the non-target tissue (), nor in the whole body dose ( and ). This treatment modality is therefore not expected to result in any significant difference in the risk for secondary malignancies, which is contrary to the risk estimated by Hall and Wuu. However, their estimations might be valid for IMRT with the serial tomotherapy due to the increased whole body dose ( and ). However, longer follow-up and larger number of patients will be necessary to conclude whether IMRT increases the number of second malignancies compared with conventional radiotherapy.
Acknowledgements
We would like to thank Dr Sven Hertzman for critically reviewing this manuscript.
References
- Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. Out-of-field photon and neutron dose equivalents from step-and-shoot intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62: 1204–16
- Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62: 1195–203
- Stovall M, Blackwell CR, Cundiff J, Novack DH, Palta JR, Wagner LK, et al. Fetal dose from radiotherapy with photon beams: Report of AAPM Radiation Therapy Committee Task Group No. 36. Med Phys 1995; 22: 63–82
- Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet 1999; 353(9149)267–72
- Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: Update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys 2006; 64: 57–62
- Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol 2000; 55: 241–9
- Glimelius B, Ask A, Bjelkengren G, Bjork-Eriksson T, Blomquist E, Johansson B, et al. Number of patients potentially eligible for proton therapy. Acta Oncol 2005; 44: 836–49
- Cella L, Lomax A, Miralbell R. Potential role of intensity modulated proton beams in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 2001; 49: 217–23
- Johansson J, Blomquist E, Montelius A, Isacsson U, Glimelius B. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol 2004; 72: 129–38
- Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol 2001; 61: 287–97
- Mu X, Bjork-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, et al. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol 2005; 44: 554–62
- Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys 2004; 58: 1596–606
- Van der Giessen PH. A simple and generally applicable method to estimate the peripheral dose in radiation teletherapy with high energy x-rays or gamma radiation. Int J Radiat Oncol Biol Phys 1996; 35: 1059–68
- Stovall M, Blackwell CR, Cundiff J, Novack DH, Palta JR, Wagner LK, et al. Erratum: “Fetal dose from radiotherapy with photon beams: Report of AAPM Radiation Therapy Committee Task Group No. 36”[Med Phys 1995;22:63–82]. Med Phys 1995; 22: 1353–4
- Jordan TJ. Central axis depth dose data for use in radiotherapy Section 5: Megavoltage X-ray beams: 2–50 MV. Br J Radiol 1996; (Suppl 25): 62–109
- Epstein RJ, Kelly SA, Cook M, Bateman A, Paddick I, Kam KC, et al. Active minimisation of radiation scatter during breast radiotherapy: Management implications for young patients with good-prognosis primary neoplasms. Radiother Oncol 1996; 40: 69–74
- Mutic S, Esthappan J, Klein EE. Peripheral dose distributions for a linear accelerator equipped with a secondary multileaf collimator and universal wedge. J Appl Clin Med Phys 2002; 3: 302–9
- Mutic S, Klein EE. A reduction in the AAPM TG-36 reported peripheral dose distributions with tertiary multileaf collimation. American Association of Physicists in Medicine Task Group 36. Int J Radiat Oncol Biol Phys 1999; 44: 947–53
- Stern RL. Peripheral dose from a linear accelerator equipped with multileaf collimation. Med Phys 1999; 26: 559–63
- Mazonakis M, Varveris H, Fasoulaki M, Damilakis J. Radiotherapy of Hodgkin's disease in early pregnancy: Embryo dose measurements. Radiother Oncol 2003; 66: 333–9
- Sharma DS, Animesh, Deshpande SS, Phurailatpam RD, Deshpande DD, Shrivastava SK, et al. Peripheral dose from uniform dynamic multileaf collimation fields: Implications for sliding window intensity-modulated radiotherapy. Br J Radiol 2006; 79(940)331–5
- Petti PL, Chuang CF, Smith V, Larson DA. Peripheral doses in CyberKnife radiosurgery. Med Phys 2006; 33: 1770–9
- Jeraj R, Mackie TR, Balog J, Olivera G, Pearson D, Kapatoes J, et al. Radiation characteristics of helical tomotherapy. Med Phys 2004; 31: 396–404
- Ramsey CR, Seibert R, Mahan SL, Desai D, Chase D. Out-of-field dosimetry measurements for a helical tomotherapy system. J Appl Clin Med Phys 2006; 7: 1–11
- Meeks SL, Paulino AC, Pennington EC, Simon JH, Skwarchuk MW, Buatti JM. In vivo determination of extra-target doses received from serial tomotherapy. Radiother Oncol 2002; 63: 217–22
- Mutic S, Low DA. Whole-body dose from tomotherapy delivery. Int J Radiat Oncol Biol Phys 1998; 42: 229–32
- Reft CS, Runkel-Muller R, Myrianthopoulos L. In vivo and phantom measurements of the secondary photon and neutron doses for prostate patients undergoing 18 MV IMRT. Med Phys 2006; 33: 3734–42
- Roy SC, Sandison GA. Shielding for neutron scattered dose to the fetus in patients treated with 18 MV x-ray beams. Med Phys 2000; 27: 1800–3
- Binns PJ, Hough JH. Secondary dose exposures during 200 MeV proton therapy. Radiat Prot Dosim 1997; 70: 441–4
- Mesoloras G, Sandison GA, Stewart RD, Farr JB, Hsi WC. Neutron scattered dose equivalent to a fetus from proton radiotherapy of the mother. Med Phys 2006; 33: 2479–90
- Polf JC, Newhauser WD. Calculations of neutron dose equivalent exposures from range-modulated proton therapy beams. Phys Med Biol 2005; 50: 3859–73
- Roy SC, Sandison GA. Scattered neutron dose equivalent to a fetus from proton therapy of the mother. Radiat Phys Chem 2004; 71: 997–8
- Schneider U, Agosteo S, Pedroni E, Besserer J. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 2002; 53: 244–51
- Yan X, Titt U, Koehler AM, Newhauser WD. Measurement of neutron dose equivalent to proton therapy patients outside of the proton radiation field. Nuclear Inst Methods Phys Res A 2002; 476: 429–34
- Agosteo S, Birattari C, Caravaggio M, Silari M, Tosi G. Secondary neutron and photon dose in proton therapy. Radiother Oncol 1998; 48: 293–305
- Jiang H, Wang B, Xu XG, Suit HD, Paganetti H. Simulation of organ-specific patient effective dose due to secondary neutrons in proton radiation treatment. Phys Med Biol 2005; 50: 4337–53
- Howell RM, Hertel NE, Wang Z, Hutchinson J, Fullerton GD. Calculation of effective dose from measurements of secondary neutron spectra and scattered photon dose from dynamic MLC IMRT for 6 MV, 15 MV, and 18 MV beam energies. Med Phys 2006; 33: 360–8
- Vanhavere F, Huyskens D, Struelens L. Peripheral neutron and gamma doses in radiotherapy with an 18 MV linear accelerator. Radiat Prot Dosimetry 2004; 110: 607–12
- Durante M, Yamada S, Ando K, Furusawa Y, Kawata T, Majima H, et al. Measurements of the equivalent whole-body dose during radiation therapy by cytogenetic methods. Phys Med Biol 1999; 44: 1289–98
- Followill D, Geis P, Boyer A. Estimates of whole-body dose equivalent produced by beam intensity modulated conformal therapy. Int J Radiat Oncol Biol Phys 1997; 38: 667–72
- Goddu S, Mutic S, Grigsby J, Santanam L, Low D. Whole-body dose for helical tomotherapy. Med Phys 2006; 33: 2150
- Verellen D, Vanhavere F. Risk assessment of radiation-induced malignancies based on whole-body equivalent dose estimates for IMRT treatment in the head and neck region. Radiother Oncol 1999; 53: 199–203
- Glimelius B, Isacsson U, Blomquist E, Grusell E, Jung B, Montelius A. Potential gains using high-energy protons for therapy of malignant tumours. Acta Oncol 1999; 38: 137–45
- Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med 1998; 128: 114–7
- Boice JD, Jr, Day NE, Andersen A, Brinton LA, Brown R, Choi NW, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst 1985; 74: 955–75
- Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 2000; 88: 398–406
- Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003; 56: 83–8
- Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol 2006; 176: 1415–9
- Aoyama H, Westerly DC, Mackie TR, Olivera GH, Bentzen SM, Patel RR, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys 2006; 64: 962–7
- Johansson J. Thesis: Comparative treatment planning in radiotherapy and clinical impact of proton relative biological effectiveness. Acta Universitatis Upsaliensis, Uppsala 2006
- Johansson J, Isacsson U, Lindman H, Montelius A, Glimelius B. Node-positive left-sided breast cancer patients after breast-conserving surgery: Potential outcomes of radiotherapy modalities and techniques. Radiother Oncol 2002; 65: 89–98
- Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier PA, et al. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol 1999; 51: 257–71
- Lomax AJ, Cella L, Weber D, Kurtz JM, Miralbell R. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003; 55: 785–92
- Mock U, Bogner J, Georg D, Auberger T, Potter R. Comparative treatment planning on localized prostate carcinoma conformal photon- versus proton-based radiotherapy. Strahlenther Onkol 2005; 181: 448–55
- Mock U, Georg D, Bogner J, Auberger T, Potter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2004; 58: 147–54
- Pirzkall A, Carol M, Lohr F, Hoss A, Wannenmacher M, Debus J. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys 2000; 48: 1371–80