Abstract
This paper deals with some of the musculo-skeletal complication that can occur after cancer treatment. In particular, we focus on Cancer Treatment Induced Bone Loss (CTIBL) and the musculo-skeletal complications that can occur in patients treated for extremity sarcoma. In addition we discuss peripheral neuropathy, musculo-skeletal pain and briefly mention some of the complications related to radiotherapy. CTIBL is mostly studied in breast cancer and prostate cancer survivors. The cause in these groups is mainly due to treatment induced hypogonadism. Other causes of CTIBL are indirect or direct cause of chemotherapy, physical inactivity and inadequate intake of vitamin D and calcium. Treatment of CTIBL consists of diet and lifestyle changes and pharmacological intervention. Extremity bone sarcomas constitute a special group since they often experience mutilating surgery and heavy combination chemotherapy. The treatment results in worse function than the normal population and the amputated usually have lower physical functioning than patients treated with limb sparing surgery (LSS). However, most studies fail to show differences in quality of life between the amputated and LSS. Most of the studies performed on musculo-skeletal sequelae have been done on survivors of childhood cancer, breast cancer or prostate cancer. More studies among the other cancer groups are needed to reveal the extent and prevalence of these complications.
Advances in diagnostic techniques and new treatment strategies have led to improved cancer cure rates and prolonged survival in adult and, in particular paediatric patients. Today close to 60% of all cancer patients in the developed world will survive for more than 5 years. Consequently, health care providers are increasingly focusing on quality of life aspects by identifying and reducing long-term toxicities in cancer survivors.
This paper outlines some of the musculo-skeletal complications that can occur in cancer survivors in general and presents, in more detail, cancer treatment induced bone loss (CTIBL) and the musculo-skeletal problems that can occur in patients treated for extremity localized sarcomas.
Peripheral neuropathy
Peripheral neuropathy is a potential side effect of chemotherapy. Despite being a neurological side effect, it is mentioned here because the neuropathy usually affects the hands and feet and may give muscle weakness and pain. Chemotherapeutic agents such as cisplatin, oxaliplatin, taxanes and vinca alkaloids are known to cause neuropathy Citation[1–3]. The symptoms usually appear during chemotherapy.
Direct damage to the nerve cell by pressure or trauma (tumour or surgery) can also cause neuropathy. The symptoms may diminish once the treatment has stopped, but they may persist for years in some patients. The incidence and prevalence of long-term peripheral neuropathy are not known Citation[1].
The clinical manifestations of peripheral neuropathy are most often reported as a distal symmetrically distributed burning, numbness, tingling, decreased or altered sensation, or increased sensitivity that may be painful Citation[2]. Symptoms of motor weakness due to peripheral neuropathy are less commonly reported.
Clinical examination demonstrates impairment of tactile sensation, altered two-point discrimination, proprioception, temperature and vibration in the distal area corresponding to the patient's clinical symptoms. Many patients may also have ataxia and demonstrate a positive Romberg sign.
Several agents that have been evaluated for their potential use as a chemoprotective agent to prevent peripheral neuropathy, but so far none have proven effective Citation[2], Citation[4].
There is currently no treatment that can reverse nerve damage; hence treatment is directed toward symptom management. Physical therapy may be helpful in providing improved strength, balance and coordination. For patients with drop-foot support for feet and ankles can be improved by orthotic devices.
Cancer treatment induced bone loss
Hormone therapy, chemotherapy, radiation therapy and surgical castration, can all directly or indirectly damage bone and lead to loss of bone mass. Bone mineral density (BMD) can be measured with several non-invasive methods such as dual energy x-ray absorptionmetry (DEXA), peripheral DEXA, quantitative computed tomography scan (QCT) and ultrasound. DEXA is currently considered as the “gold standard” when performed at the femoral neck or total hip Citation[5–8]. Bone densimetry results are often reported as t-scores, which represent the difference in the number of standard deviations (SD) between the individual's BMD and the mean value for a group of young adults of the same sex (and in some cases the same race). The World Health Organization has established diagnostic categories of bone loss based on BMD measurements Citation[9]. Normal bone mass is defined by the World Health Organization as BMD within one SD of young adult mean (t-score ≥ 1); osteopenia as increased bone loss, with bone mass between 1 and 2.5 S.D. below normal (t-score, <1 and >2.5); and osteoporosis as bone mass > 2.5 S.D. below normal (t-score > 2.5).
Severe osteoporosis is defined as osteoporosis in the presence of one or more fragility fractures ().
Table I. Classification of osteoporosis.
For every SD by which BMD is below peak bone mass, fracture risk approximately doubles Citation[10], Citation[11].
Normal aging gives bone loss primarily as a result of hypogonadism. CTIBL is mostly studied in Breast Cancer Survivors (BCS) and Prostate Cancer Survivors (PCS) with the focus on therapies that induce hypogonadism Citation[5], Citation[6], Citation[8], Citation[12–16]. There is little information in the literature on the risk, incidence and detection of osteoporosis in other population of cancer survivors Citation[6].
There have also been some studies in childhood cancer survivors. However, these studies show diverse results and it is hard to draw any strict conclusions Citation[11], Citation[17–24]. One of the larger studies, where Mandel et al. compared 106 survivors of acute lymfoblastic leukaemia (ALL) in childhood with age matched normal controls, showed that the survivors of childhood ALL as a whole recover normal BMD. A minority of the patients who received an accumulated Methotrexate dose greater than 40 g/m2 or a total corticosteroid dose of greater than 9 g/m2 did not recover normal BMD Citation[17]. Other studies have shown that patients treated for brain tumour, bone sarcoma etc. might show low BMD years after therapy Citation[18], Citation[23], Citation[24].
Causes of CTIBL
The primary cause of CTIBL is hypogonadism induced by chemotherapy, hormone therapy, surgical castration or radiation therapy ().
Table II. Causes of cancer treatment induced bone loss.
In BCS, hormone therapies given to induce premature menopause or further reduce circulating estrogen levels are a common cause of CTIBL. Tamoxifen has been shown to both cause and prevent bone loss, depending on the menopausal status of the woman. In premenopausal women, with high estrogen levels, it works as a bone antagonist, whereas in postmenopausal women, with low estrogen levels, it works as a bone agonist and, hence, seemingly protects against bone loss Citation[12], Citation[25], Citation[26]. Aromatase inhibitors (AI) lack estrogen agonist or antagonist activity. By decreasing aromatase activity and inhibiting the conversion of adrenal androgens to estrogen, they reduce circulating and tissue levels of estrogen which might accelerate bone loss Citation[6], Citation[12], Citation[15], Citation[27], Citation[28].
Androgen deprivation therapy (ADT) in prostate cancer is a potent induction of hypogonadism. The exact mechanism whereby hypogonadism induces CTIBL associated with prostate cancer is unknown. In hypogonadal prostate cancer patients, the reduction of circulating testosterone and estrogen levels causes a decrease in osteoblastic bone formation and an increase in osteoclastic bone resorption, leading to accelerated bone loss Citation[6], Citation[8], Citation[16].
Other causes of CTIBL are indirect or direct causes of therapy. Some chemotherapeutics are known to induce bone loss. Methotrexate increases bone resorption and reduces bone formation. In addition it inhibits matrix mineralization and thereby further reduces bone formation Citation[6], Citation[15], Citation[29], Citation[30].
Cyclophosphamide inhibits bone formation and bone resorption by directly arresting the cell division of preosteoblast and osteoclast which leads to decreased number of osteoblasts and osteoclasts on bone surfaces Citation[6], Citation[15]. Ifosfamide might cause bone loss indirectly through renal tubular nephrotoxicity which can result in hypophosphatemia that may lead to defective mineralization and demineralization of bone. Bone formation may also be inhibited in absence of severe renal dysfunction Citation[6], Citation[15], Citation[29].
In vitro studies of doxorubicin have shown that the drug inhibits proliferation and differentiation of osteoblasts and selectively reduces the rate of bone formation by altering the interaction of parathyroid hormone with the osteoblast reseptor Citation[6], Citation[15].
Glucocorticoids, commonly used as a pain adjuvant, palliative agent, antiemetic or as a part of the treatment, inhibit the activity of osteoblasts and hence the bone formation is decreased. In addition the intestinal calcium absorption is inhibited and the renal excretion of calcium is increased. The negative calcium balance can increase the parathyroid hormone (PTH) secretion. PTH acts on osteoclasts to increase bone resorption. Corticosteroids can also suppress the secretion of gonadal hormones and growth hormone Citation[31].
Inactivity can also cause bone loss as can inadequate intake of calcium and vitamin D Citation[10], Citation[32], Citation[33].
Prevention and treatment of CTIBL
The frequency of CTIBL in BCS is not known. It depends on the treatment received. In PCS receiving ADT, annual bone loss is about 2–9% Citation[5], Citation[14].There is seemingly no recovery after therapy is ceased. In other cancer groups, prospective studies on bone loss are lacking, so the incidence in these groups are not known.
There is no routine screening for osteoporosis after cancer treatment. The American Society of clinical oncology (ASCO) has published guidelines for breast cancer survivors Citation[27]. The Norwegian Breast Cancer Group (NBCG) recommend that everyone receiving AI shall have BMD measured before start, after one year of therapy and thereafter every 2 years until AI is discontinued. Osteopeni/osteoporosis will lead to start of bisphosfonates (www.nbcg.net).
To our knowledge there are no published guidelines on screening PCS or any other patient population at risk for CTIBL. Diamond et al. have published recommendations for screening PCS, but no organization has reached a consensus on screening this population Citation[34].
The treatment of CTIBL is based on advices given to the general population. Diet and life style changes include adequate calcium and vitamin D intake. Weight bearing exercises, such as running and weight training have been shown to increase BMD. Less vigorous weight bearing exercise, such as walking or low impact aerobic protects against further bone loss Citation[5], Citation[32]. Exercise also improves the muscle strength.
Cessation of smoking and reducing alcohol intake might also prevent further bone loss. Several studies have demonstrated significant correlations between smoking and decreased BMD Citation[5], Citation[13], Citation[32].
When it comes to pharmacological therapy, hormone replacement therapy can be given to people with hypogonadism. This, however, is clearly contraindicated in BCS and PCS. The most used pharmacological therapy is bisphosphonates in tablet formulation with the additional supplement of Vitamin D and calciumCitation[5], Citation[8], Citation[15], Citation[16], Citation[35]. This class of drugs is widely used for treatment of postmenopausal, steroid induced and male osteoporosis. The use of intravenous bisphosphonates is still under investigation; in particular as to what time interval between infusions that is optimal. There is an ongoing study in patients with breast cancer that receive the aromatase inhibitor Letrozol (Femar); the Z-fast study. In the Z-fast study, where some of the patients receive zoledronic acid (Zometa) upfront and some delayed, preliminary data suggests that getting Zoledronic upfront prevents CTIBL Citation[36].
Complication due to radiotherapy
Among the several known complications to radiotherapy, muscle atrophy, fibrosis, fractures and limb length discrepancy are worrisome Citation[37–39].
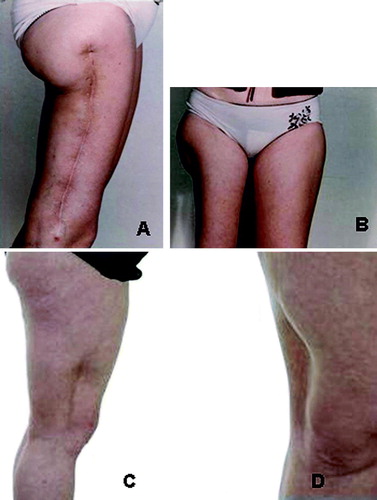
In the case of soft-tissue sarcoma patients the current use of post-operative radiotherapy in marginally resected deep high-grade tumours may result in an improved functional outcome compared to extensive compartmental surgery () without comprising local control rates and survival Citation[40–43].
Figure 1. Comparison of wide surgery alone and a marginal resection with post-operative radiotherapy in high-grade soft-tissue sarcoma patients: A and B: Patient with functional sequelae following compartmental surgery alone C and D: Patient with preserved function treated by post-operative radiotherapy (2 Gy x 25 CT based doseplanning) with shielding of > 50% of the extremity circumference.
Scoliosis/Kyphosis
In patients given radiotherapy to the trunk, scoliosis and or kyphosis might occur. It is mostly seen in survivors of childhood Wilm's tumour or neuroblastoma. In older series the incidence was up to 70% Citation[44], but after lowering radiation doses, and also applying symmetrical radiation fields to the entire width of the columna, later studies report of lower incidence. Paulino et al. found in his study of late effects after treatment for Wilm's tumour that children treated with radiation doses below 24 Gy had a lower incidence of scoliosis compared with those who received more than 24 Gy. There was also a suggestion that the incidence is further reduced in patients who received 10–12 Gy. He also showed that the incidence seemed to progress with time from treatment Citation[45].
Scoliosis may also be seen in those who have been treated with surgical laminectomi only for intraspinal tumours Citation[46].
When it comes to treatment some will receive bracing for their scoliosis and a few need surgery.
Musculo-skeletal pain
Pain in cancer survivors is complex. Some patients report of more general pain compared to people with no history of cancer Citation[1], Citation[47].There are sensory pain, pain in the radiated area and some reports of muscular pain. The cause and the extent of the pain are often not reported. Hudson et al. and found that survivors of bone tumours were more likely to have cancer related pain than other survivors of childhood tumours Citation[48].
Some studies indicate that higher radiation doses (above 60 Gy) give more pain, and also larger dose per fraction may lead to damage to the muscle and soft tissue, which can give pain. Progression of injury may continue for as long as 10 years Citation[49]. The current trend to reduce the volume of normal tissue irradiated has the potential of keeping complications from becoming a major clinical problem.
Surgery may also cause pain either because of nerve damage or physical impairment. An unpublished study of extremity bone sarcoma survivors, shows that those with lower functional score, report of more muscle pain than the other survivors (Aksnes et al., unpublished results)
Keating et al. studied older cancer survivors (>55 years old) and compared them to individuals with no history of cancer. She found that the cancer survivors were more likely to report pain (36% vs. 29%, p = 0.005) and more suffered from arthritis (69% vs. 59%, p < 0.001) Citation[50].
Physical impairment
Limb-sparing surgery versus amputation
Patients treated for extremity localized sarcomas are likely to experience physical impairment after treatment. This is a patient group that often get mutilating surgery and massive chemotherapy. During the past 25 years the amputation rate has gone down and more sophisticated limb sparing techniques have developed. Currently, approximately 90% of primary bone-sarcoma patients can avoid amputation Citation[51], Citation[52], seemingly without a higher rate of local relapses Citation[53], Citation[54]. This is due to both improvements in diagnostic radiology and refined surgical techniques, as well as the use of neo-adjuvant combination chemotherapy; the current strategy advocated at most centres. Such pre-operative therapy acts as a “protective umbrella” when, as in most cases, the tumour responds to chemotherapy.
The long-term implications of limb salvage and amputation in paediatric patients with primary tumours in the lower extremity has been reviewed Citation[55]. Obviously, the patient's acute stress is lower at primary diagnosis when a message of limb sparing can be conveyed. Furthermore, there is a general acceptance that both early complication and the need for re-operations during years of follow-up are increased in the limb-sparing cohort Citation[54], Citation[56]. In an ongoing long-term follow-up study among extremity-localized Ewing's sarcoma or osteosarcoma survivors in Norway, 56% of the limb-sparing patients had undergone more than one operation compared to 10% of the amputees. The median numbers of surgical procedures were two among the limb sparing with a range of 1–6 (Aksnes et al., unpublished results).
Whether to amputate or perform limb- salvage rises several important questions:
If histological margins turn out to be inadequate due to too much eager to save the limb, will this reduce the probability for survival?
Will a local relapse per se give rise to secondary metastases or is it just a sign of an aggressive biology of the sarcoma?
Will early complications of limb-salvage result in delayed chemotherapy with reduced dose-intensity? Will this compromise survival?
How does the early and late morbidity compare between amputation and limb salvage?
Will a limb-sparing surgery result in improved function compared with amputation?
Are there any differences in overall quality of life (QoL) between the two options?
Aksnes et al. have compared quality of life, mental distress and fatigue between bone sarcoma survivors and testicular cancer survivors, Hodgkin cancer survivors and norm population in Norway. The bone sarcoma patients show poorer physical function than the other survivor groups and norm, but there were no differences in overall QoL, mental distress or fatigue (unpublished observations). The extremity bone tumour survivors seemingly seem to adapt to their new situation and cope with their handicap. This may be due to ‘response shift’ in the survivors. Response shift is defined as a change in one's self-evaluation, internal standards, values or conceptualisations as a result of health state changes. Citation[67], Citation[68]. So even though they may have a quite visible handicap after i.e. amputation, it has been a part of their every day life and does not affect their quality of life.
Conclusion
There are several musculo-skeletal complications that can occur after cancer treatment. In this paper we have dealt with some of them. Many of the studies performed have focused on problems occurring in survivors of childhood cancer, breast cancer and prostate cancer. More studies are needed among the other cancer groups to reveal the severity and extent of musculo-skeletal complications and how to prevent/minimize these in the future without reduction of the cure rate.
References
- Polomano RC, Farrar JT. Pain and neuropathy in cancer survivors: Surgery, radiation, and chemotherapy can cause pain; research could improve its detection and treatment. Am J Nurs 2006; 106(Suppl 3)39–47
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006; 33: 15–49
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2006; 249: 9–17
- Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy:pathogenesis and emerging therapies. Support Care Cancer 2004; 12: 619–25
- Bae DC, Stein BS. The diagnosis and treatment of osteoporosis in men on androgen deprivation therapy for advanced carcinoma of the prostate. J Urol 2004; 172: 2137–44
- Michaud LB, Goodin S. Cancer-treatment-induced bone loss, part 1. Am J Health-Syst Pharm 2006; 63: 419–30
- Baroncelli GI, Bertelloni S, Sodini F, Saggese G. Osteoporosis in children and adolescents: Etiology and management. Paediatr Drugs 2005; 7: 295–323
- Hoff AO, Gagel RF. Osteoporosis in breast and prostate cancer survivors. Oncology 2005; 19: 651–8
- WHO scientific group. Prevention and Management of Osteoporosis. 2003.
- Chien AJ, Goss PE. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol 2006; 24: 5305–12
- Kaste SC, Chesney RW, Hudson MM, Lustig RH, Rose SR, Carbone LD. Bone mineral status during and after therapy of childhood cancer: An increasing population with multiple risk factors for impaired bone health. J Bone Mineral Res 1999; 14: 2010–2
- Fontanges E, Fontana A, Delmas P. Osteoporosis and breast cancer. Joint Bone Spine 2004; 71: 102–10
- Hawkins R. Osteoporosis. Am J Nurs 2006; 106(Suppl 3)78–82
- Holmes-Walker DJ, Woo H, Gurney H, Do VT, Chipps DR. Maintaining bone health in patients with prostate cancer. Med J Aust 2006; 184: 176–9
- Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: Pathogenesis and management. J Clin Oncol 2000; 18: 1570–93
- Smith MR. Diagnosis and management of treatment-related osteoporosis in men with prostate carcinoma. Cancer Supplement 2003; 97: 789–95
- Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol 2004; 22: 1215–20
- Holzer G, Krepler P, Koschat MA, Grampp S, Dominkus M, Kotz R. Bone mineral density in long-term survivors of highly malignant osteosarcoma. J Bone Joint Surg (Br) 2003; 85-B: 231–7
- Kaste SC. Bone-mineral density deficits from childhood cancer and its therapy. A review of at-risk patient cohorts and available imaging methods. Pediatr Radiol 2004; 34: 373–8
- Vassilopoulou-Sellin R, Brosnan P, Delpassand A, Zietz H, Klein MJ, Jaffe N. Osteopenia in young adult survivors of childhood cancer. Med Pediatr Oncol 1999; 32: 272–8
- Haddy TB, Mosher RB, Reaman GH. Osteoporosis in survivors of acute lymphoblastic leukemia. Oncologist 2001; 6: 278–85
- Othman F, Guo CY, Webber C, Atkinson SA, Barr RD. Osteopenia in survivors of Wilm's tumor. Int J Oncol 2002; 20: 827–33
- Barr RD, Simpson T, Webber CE, Gill GJ, Hay J, Eves M, et al. Osteopenia in children surviving brain tumours. Eur J Cancer 1998; 34: 873–7
- Odame I, Duckworth J, Talsma D, Beaumont L, Furlong W, Webber C, et al. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer 2006; 46: 357–62
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Eng J Med 1992; 326: 852–6
- Resch A, Biber E, Seifert M, Resch H. Evidence that tamoxifen preserves bone density in late postmenopausal women with breast cancer. Acta Oncol 1998; 37: 661–4
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology: American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003; 21: 4042–57
- Eastell R, Hannon R. Long-term effects of aromatase inhibitors on bone. J Steroid Biochem Mol Biol 2005; 95: 151–4
- van Leeuwen BL, Kamps WA, Jansen HW, Hoekstra HJ. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev 2000; 26: 363–76
- Theriault RL. Pathophysiology and implications of cancer treatment-induced bone loss. Oncology 2004; 18(5 Suppl 3)11–5
- Bianchi ML. Glucorticoids and bone: Some general remarks and some special observations in pediatric patients. Calcif Tissue Int 2002; 70: 384–90
- Conde FA, Aronson WJ. Risk factors for male osteoporosis. Urol Oncol: Sem Orig Invest 2003; 21: 380–3
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12: 6315–9
- Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: Recommendations for diagnosis and therapies. Cancer 2004; 100: 892–9
- Michaud LB, Goodin S. Cancer-treatment-induced bone loss, part 2. Am J Health-Syst Pharm 2006; 63: 534–46
- Brufsky, A. Management of cancer-treatment-induced bone loss in postmenopausal women undergoing adjuvant breast cancer therapy: A Z-FAST update. Semin Oncol 2006;33(Suppl 7):s13–s17.
- Butler MS, Robertson WW, Jr, Rate W, D'Angio GJ, Drummond DS. Skeletal sequelae of radiation therapy for malignant childhood tumors. Clin Orthop 1990; 251: 235–40
- Fletcher BD. Effects of pediatric cancer therapy on the musculoskeletal system. Pediatr Radiol 1997; 27: 623–36
- Parisi MT, Fahmy JL, Kaminsky CK, Malogolowkin MH. Complications of cancer therapy in children: A radiologist's guide. Radiographics 1999; 19: 283–96
- Ballo MT, Zagars GK. Radiation therapy for soft tissue sarcoma. Surg Oncol Clin North Am 2003; 12: 449–67
- DeLaney TF, Thomas F. Optimizing radiation therapy and post-treatment function in the management of extremity soft tissue sarcoma. Curr Treat Options Oncol 2004; 5: 463–76
- O'Sullivan B, Ward I, Catton C. Recent advances in radiotherapy for soft-tissue sarcoma. Curr Oncol Rep 2003; 5: 274–81
- Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol 2003; 42: 516–31
- Riseborough EJ, Grabias SL, Burton RI, Jaffe N. Skeletal alterations following irradiation for Wilms’ tumor: With particular reference to scoliosis and kyphosis. J Bone Joint Surg (Am) 1976; 58: 526–36
- Paulino AC, Wen BC, Brown CK, Tannous R, Mayr NA, Zhen WK, et al. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys 2004; 60: 265–74
- Paulino AC, Fowler BZ. Risk factors for scoliosis in children with neuroblastoma. Int J Radiat Oncol Biol Phys 2005; 61: 865–9
- Lyne, ME, Coyne, PJ, Watson, AC. Pain management issues for cancer survivors. Cancer Practice 2002;10(Suppl 1):s27–s32.
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA 2003; 290: 1583–92
- Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys 1995; 31: 1309–18
- Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc 2005; 53: 2145–52
- Aksnes LH, Hall KS, Folleraas G, Stenwig AE, Bjerkehagen B, Taksdal I, et al. Management of high-grade bone sarcomas over two decades: The Norwegian Radium Hospital experience. Acta Oncol 2006; 45: 38–46
- Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: An updated report. J Clin Oncol 2000; 18: 4016–27
- Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol 2005; 6: 85–92
- Veth R, van HR, Pruszczynski M, Hoogenhout J, Schreuder B, Wobbes T. Limb salvage in musculoskeletal oncology. Lancet Oncol 2003; 4: 343–50
- Nagarajan R, Neglia JP, Clohisy DR, Robison LL. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: What are the long-term implications?. J Clin Oncol 2002; 20: 4493–501
- Wilkins RM, Miller CM. Reoperation after limb preservation surgery for sarcomas of the knee in children. Clinical Orthop 2003; 412: 153–61
- Eiser C, Darlington A-S.E, Stride CB, Grimer RJ. Quality of life implication as a consequence of surgery: Limb salvage, primary and secondary amputation. Sarcoma 2001; 5: 189–95
- Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: A matched case-control study. Arch Phys Med Rehabil 1999; 80: 615–8
- Grimer RJ, Taminiau AM, Cannon SR. Surgical outcomes in osteosarcoma. J Bone Joint Surg (Br) 2002; 84-B: 395–400
- Hopyan SMPF, Tan JWM, Graham HKM, Torode IPF. Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Orthopaed 2006; 26: 405–8
- Nagarajan R, Clohisy DR, Neglia JP, Yasui Y, Mitby PA, Sklar C, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing's sarcoma: The Childhood Cancer Survivor Study. Br J Cancer 2004; 91: 1858–65
- Postma A, Kingma A, De Ruiter JH, Schraffordt KH, Veth RP, Goeken LN, et al. Quality of life in bone tumor patients comparing limb salvage and amputation of the lower extremity. J Surg Oncol 1992; 51: 47–51
- Refaat YM, Gunnoe JB, Hornicek FJM, Mankin HJM. Comparison of quality of life after amputation or limb salvage. Clinical Orthop 2002; 397: 298–305
- Renard AJ, Veth RP, Schreuder HW, van Loon CJ, Koops HS, van H, Jr. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol 2000; 73: 198–205
- Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am 1994; 76: 649–56
- Zahlten-Hinguranage A, Bernd L, Ewerbeck V, Sabo D. Equal quality of life after limb-sparing or ablative surgery for lower extremity sarcomas. Br J Cancer 2004; 91: 1012–4
- Sprangers MA. Quality-of-life assessment in oncology. Achievements and challenges. Acta Oncol 2002; 41: 229–37
- Sprangers MA, Van Dam FS, Broersen J, Lodder L, Wever L, Visser MR, et al. Revealing response shift in longitudinal research on fatigue–the use of the thentest approach. Acta Oncol 1999; 38: 709–18