Abstract
Background and Purpose. Fatigue is one of the most common and troubling symptoms in cancer survivors. In this paper we review information about cancer related fatigue in survivors of breast cancer and Hodgkin's disease, discuss some of the potential biological mechanisms for this problem in cancer survivors, and briefly discuss potential interventions. Findings. Cancer-related fatigue persists long after cancer treatments end, and is associated with more intensive treatments (combined chemotherapy and radiation therapy) in these cancers. Fatigue prior to the onset of treatment is a strong predictor of persistent fatigue. Studies in breast cancer survivors suggest elevated levels of pro-inflammatory cytokines in association with persistent fatigue, as well as abnormalities in the hypothalamic-pituitary axis. Psychosocial and physical activity interventions have been shown in some studies to alleviate fatigue. Conclusions. Recognizing the syndrome of cancer-related fatigue is a high priority for the many cancer survivors who continue to experience this complaint as a chronic health problem.
Fatigue is the most common side effect of cancer and its treatment, with prevalence estimates ranging from 60–96% for patients who are on active cancer treatment Citation[1]. Fatigue is associated with all treatment modalities (cytotoxic chemotherapy, radiation therapy, surgery, and biotherapies), and may be the presenting symptom at cancer diagnosis Citation[2]. Fatigue is also very common in patients with advancing cancer nearing death. Alleviation of pain and suffering are important goals of cancer care, yet strategies to alleviate and manage fatigue have been more limited.
A growing body of research indicates that fatigue may endure for months or years after successful treatment completion Citation[3–5], causing significant impairment in overall quality of life Citation[6], Citation[7]. Indeed, a large survey study found that cancer patients felt that fatigue adversely affected their daily lives more than pain or other symptoms Citation[8]. There is growing consensus regarding a case definition for identifying cancer-related fatigue, which some have defined as a persistent and subjective sense of tiredness that interferes with usual functioning Citation[6], Citation[7], Citation[9].
In this manuscript, we review research on the prevalence and correlates of fatigue in two survivor populations, breast cancer survivors and Hodgkin's disease survivors, for whom there exists a substantial database. We also present early evidence for potential biological mechanisms underlying the development of fatigue, and briefly describe treatment strategies that have been used to manage fatigue symptoms.
Fatigue after cancer and its treatments
Prevalence and correlates of fatigue in breast cancer survivors
Fatigue is one of the most prevalent and disabling side effects of breast cancer treatment Citation[7], Citation[10]. It is elevated in breast cancer survivors relative to age-matched healthy controls Citation[11], Citation[12], with approximately 30% of survivors reporting moderate to severe symptoms of fatigue Citation[13–15]. Recent work conducted by our group indicates that fatigue may endure for up to 10 years after breast cancer diagnosis Citation[5]. In one study, fatigue reported by women who had received chemotherapy was 50% higher than women with no history of breast cancer, indicating the clinical significance of this symptom Citation[12]. It negatively impacts work, social relations, and daily activities, and causes significant impairment in physical function and overall quality of life in breast cancer survivors Citation[11–13]. Fatigue is also associated with declines in physical activity among breast cancer patients and survivors Citation[16], Citation[17].
Fatigue is a multidimensional symptom and may be influenced by psychological, physical, and biological factors. Among breast cancer patients and survivors, fatigue is strongly correlated with psychological distress and depression Citation[11], Citation[13], Citation[18], Citation[19]. Depressed mood, pain, and sleep disturbance were the strongest correlates of fatigue in our large study of breast cancer survivors assessed at 1–5 years post diagnosis Citation[13]. In a longitudinal follow-up of this cohort, depressed mood continued to predict fatigue at 5–10 years post diagnosis, as did cardiovascular problems and type of cancer treatment received Citation[5]. In particular, women treated with either radiation or chemotherapy showed a small improvement in fatigue symptoms relative to women who received combined therapy. Recent research suggests that coping strategies used to manage fatigue and patients’ perceived self-efficacy in controlling this symptom may also influence fatigue levels. For example, greater use of catastrophizing as a coping strategy was associated with more severe fatigue in breast cancer survivors Citation[12]. Fatigued breast cancer survivors also report lower levels of control over their symptoms than non-fatigued patients Citation[15]. In contrast, women who cope actively by maintaining their activity levels report decreased fatigue during and after chemotherapy Citation[16].
Prevalence and correlates of fatigue in Hodgkin's disease patients and survivors
In one of the earliest studies of Hodgkin's disease (HD) survivors, Fobair et al. Citation[20] reported that ongoing fatigue was a major concern for 37% of 403 survivors. Fatigue was influenced by age, time since diagnosis, stage of disease and type of treatment (younger age, longer time since diagnosis, earlier stage, and radiation therapy without chemotherapy were all significantly better). Fatigued survivors also reported higher rates of depression, consistent with results seen among breast cancer survivors. Other concerns identified in these survivors were marital disruption, problems with infertility, and less interest in sexual activity. In addition, 29% of this sample was unemployed, with 18% currently looking for work Citation[20].
More recent cross-sectional studies examining HD survivors either treated on clinical trials or from large treatment centers Citation[21–24], have largely confirmed the findings of Fobair et al. Citation[20] with regard to the prevalence of fatigue among HD survivors. In addition, these studies also noted that HD survivors performed more poorly on measures of physical and psychosocial function in comparison to either patients with acute leukemia, testicular cancer or healthy population samples. These studies suggested a relationship of fatigue and physical performance to the intensity of treatments; however, their retrospective and uncontrolled design limits the ability to determine causality.
A recent longitudinal follow-up study in HD survivors did not find a relationship between treatment intensity and chronic fatigue Citation[25], but did note an association with B symptoms at diagnosis with chronic fatigue. Further study of this same sample found that quality of life was significantly reduced in HD survivors with fatigue compared to the general population Citation[26]. Similar findings were reported in a case control study from the German Hodgkin Lymphoma Study Group Citation[27]. Finally, in a sibling control study from Ng et al., there was a modest difference in fatigue between HD survivors and their siblingsCitation[28]. A significant association was found between fatigue and cardiac disease in this sample as well as an association between tobacco use and fatigue Citation[28].
Recently, Ganz et al. Citation[2] reported on the results of a prospective study of quality of life and symptoms in patients with early stage HD treated on a clinical trial comparing subtotal nodal irradiation to combined modality therapy Citation[29]. Of note, prior to treatment with any cancer therapy, these patients had scores on the SF-36 Vitality scale that were more than a half standard deviation below the mean of the general population. Energy level diminished significantly with treatment, and was worse with the combined modality treatment. However, induction of complete remission did not lead to normalization of energy level Citation[2]. By one year after randomization, SF-36 Vitality scores had returned to the mean baseline level and remained at the same level through 2 years post-treatment. In a subsequent analysis, examining predictors of energy level at one year after treatment, baseline vitality score was the strongest predictor of subsequent score Citation[30]. Only 23% of patients experienced a significant improvement in energy with treatment while 32% reported poorer energy and 45% remained about the same. These findings suggest that the mechanism of fatigue in HD may be related to pre-existing biological factors, and may not be entirely caused by treatment.
Immune factors in cancer-related fatigue
The role of proinflammatory cytokines and the cytokine network in cancer-related fatigue has received recent attention. Proinflammatory cytokines interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) may be released as part of the host response to the tumor or in response to tissue damage, infection, or depletion of immune cell subsets related to cancer treatment Citation[31], Citation[32]. These cytokines act on the central nervous system to induce fatigue and other “sickness behavior” (e.g., decreased activity, sleep disturbance, depressed mood] Citation[33], Citation[34], suggesting a possible biological mechanism for cancer-related fatigue Citation[35]. There is preliminary evidence that proinflammatory cytokines are associated with fatigue during cancer treatment. In research conducted with breast cancer patients undergoing radiation therapy, we found that increases in fatigue were associated with increases in serum levels of IL-1β and IL-6 Citation[36]. Similarly, Greenberg et al. Citation[37] found that fatigue and serum levels of IL-1β both increased in prostate cancer patients receiving radiation therapy.
Research conducted by our laboratory indicates that proinflammatory cytokines may also play a role in persistent post-treatment fatigue Citation[17], Citation[38]. In two independent cohorts, we have shown that fatigued breast cancer survivors have elevations in circulating markers of proinflammatory cytokine activity as well as increased intracellular production of proinflammatory cytokines by monocytes in response to LPS stimulation Citation[17], Citation[38]. Fatigued survivors also showed alterations in T cell homeostasis, including elevated numbers of CD4+ “helper” T lymphocytes and T lymphocytes with an “activated effector cell” phenotype (CD56+ and CD62L + ) Citation[39]. These results suggest aberrant immunologic activity may induce cytokine alterations that subsequently impact CNS function to produce fatigue; however, the basis for this aberrant immunologic activity is not yet known. Persistent inflammatory activity in breast cancer survivors may also stem from alterations in immune regulatory systems, including the hypothalamic-pituitary-adrenal axis. We have shown that breast cancer survivors with persistent fatigue have lower levels of morning serum cortisol Citation[17], flatter diurnal cortisol slopes Citation[40], and show a blunted cortisol response to experimental stress Citation[41]. Moreover, alterations in cortisol responsiveness are directly correlated with alterations in proinflammatory cytokine production.
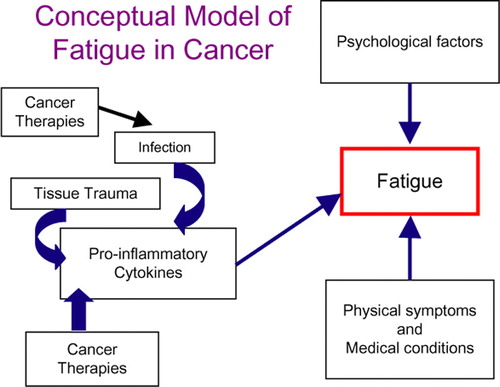
Although the literature suggests that proinflammatory cytokines may contribute to fatigue during and after treatment, research in this area is still limited to a handful of small sample studies, with only one study focusing on breast cancer patients undergoing chemotherapy Citation[42], Citation[43]. Moreover, none have followed patients from treatment onset into the post-treatment period, nor have they examined the role of pre-treatment status on subsequent immune and behavioral outcomes. Indeed, the influence of pre-treatment factors on long-term outcomes has received minimal attention, despite evidence that pre-treatment status predicts behavioral and physiological responses to cancer treatment. Longitudinal studies evaluating patients before, during, and after treatment are essential for defining the immune system's role in cancer-related fatigue, and for identifying pre-treatment factors that increase the risk for negative outcomes Citation[44]. In , we propose a conceptual model for understanding biological and psychological factors that may influence the development of fatigue in cancer patients.
Figure 1. This figure describes several etiologies for the production of pro-inflammatory cytokines in cancer patients. In their presence, along with psychological factors (depression, maladaptive coping) and physical symptoms and conditions (pain, arthritis, cardiovascular disease), survivors may experience ongoing chronic fatigue.
Interventions to address fatigue in cancer patients
A growing number of controlled intervention studies have specifically targeted fatigue in cancer patients. Most of this research has focused on exercise and has shown consistently positive results. A recent review found that all of the published exercise trials demonstrated lower levels of fatigue in cancer patients who exercised compared to control or comparison groups Citation[45]. Positive effects were demonstrated across a range of exercise programs, from home-based walking programs to supervised laboratory regimens, and across a range of cancer populations. Aerobic exercise was particularly effective, with fatigue levels approximately 40–50% lower in exercising subjects. Although most trials have been conducted with patients undergoing cancer treatment, beneficial effects have also been observed in research conducted with cancer survivors Citation[46]. Work underway in our laboratory will examine the efficacy of an Iyengar yoga intervention to alleviate for cancer-related fatigue in breast cancer survivors, with a detailed plan to investigate immune correlates of the intervention.
Psychosocial interventions have also shown beneficial effects on fatigue. For example, an educational group intervention designed to provide information about cancer and ways to manage the disease had positive effects on vitality and physical functioning in women undergoing treatment for breast cancer Citation[47], with the beneficial effects of treatment on vitality maintained over a 3-year follow-up Citation[48]. Similarly, a psycho-educational group intervention emphasizing patient education and coping skills training led to improvements in fatigue, vigor, and depressed mood among patients with malignant melanoma Citation[49]. Other forms of group therapy (i.e., supportive expressive group therapy) and individual therapy have also shown beneficial effects on fatigue Citation[50], Citation[51]. Effective treatments for fatigue do not necessarily require in-person interaction; for example, a patient self-administered form of stress management training demonstrated beneficial effects on vitality, physical function, and mental health among breast cancer patients undergoing chemotherapy Citation[52]. In a recent trial testing two different interventions to facilitate recovery after breast cancer treatment, Stanton et al. Citation[53] found that a peer-modeling video demonstrated improved recovery of energy in the 6 months following receipt of the video.
Overall, these results provide strong evidence that exercise interventions lead to improvements in cancer-related fatigue, although the mechanisms for these effects have not been determined. There is also compelling evidence that psychosocial interventions may improve energy and other aspects of mental and physical function in cancer patients. Few studies have examined pharmacologic treatments for cancer-related fatigue, other than erythropoietin; however, preliminary evidence suggests that psychostimulants may be effective for patients with advanced cancer.
Conclusions
Fatigue is one of the most common and troubling symptoms in cancer survivors. The biological mechanisms underlying this problem are beginning to be elucidated. While strongly associated with pain and psychological distress, a substantial number of survivors have neither of these complaints and experience fatigue as an isolated problem. Studies in survivors of breast cancer and Hodgkin's disease are important models for studies of other cancer sites. While the specific etiological mechanisms of fatigue may differ by cancer site, commonalities are likely to be present. With the growing number of cancer survivors, there is increased interest in developing interventions to alleviate fatigue, as it may interfere with work, social activities and enjoyment of life. A number of research groups are actively investigating the etiology of cancer-related fatigue and this should contribute to our understanding of this problem.
References
- Wagner LI, Cella D. Fatigue and cancer: Causes, prevalence and treatment approaches. Br J Cancer 2004; 91: 822–8
- Ganz PA, Moinpour CM, Pauler DK, Kornblith AB, Gaynor ER, Balcerzak SP, et al. Health status and quality of life in patients with early-stage Hodgkin's disease treated on Southwest Oncology Group Study 9133. J Clin Oncol 2003; 21: 3512–9
- Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat 1996; 39: 261–73
- Jacobsen PB, Stein K. Is fatigue a long-term side effect of breast cancer treatment?. Cancer Control 1999; 6: 256–63
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer 2006; 106: 751–8
- Mitchell SA, Berger AM. Cancer-related fatigue: The evidence base for assessment and management. Cancer J 2006; 12: 374–87
- Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol 2005; 23: 6613–22
- Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol 1997; 34(3 Suppl 2)4–12
- Jacobsen PB. Assessment of fatigue in cancer patients. J Natl Cancer Inst Monographs 2004; 32: 93–7
- Sadler IJ, Jacobsen PB. Progress in understanding fatigue associated with breast cancer treatment. Cancer Invest 2001; 19: 723–31
- Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: A controlled comparison. J Behav Med 1998; 21: 1–18
- Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol 1998; 16: 1689–96
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol 2000; 18: 743–53
- Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol 1998; 16: 1380–7
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur J Cancer 2002; 38: 27–43
- Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study. Oncol Nurs Forum 2000; 27: 1443–8
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 2002; 64: 604–11
- Smets EM, Visser MR, Willems-Groot AF, Garssen B, Oldenburger F, van Tienhoven G, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. Br J Cancer 1998; 78: 899–906
- Goldstein D, Bennett B, Friedlander M, Davenport T, Hickie I, Lloyd A. Fatigue states after cancer treatment occur both in association with, and independent of, mood disorder: a longitudinal study. BMC Cancer 2006; 6: 240
- Fobair P, Hoppe RT, Bloom J, Cox R, Varghese A, Spiegel D. Psychosocial problems among survivors of Hodgkin's disease. J Clin Oncol 1986; 4: 805–14
- Knobel H, Havard LJ, Brit LM, Forfang K, Nome O, Kaasa S. Late medical complications and fatigue in Hodgkin's disease survivors. J Clin Oncol 2001; 19: 3226–33
- Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. Hodgkin's disease survivors more fatigued than the general population. J Clin Oncol 1999; 17: 253–61
- Loge JH, Abrahamsen AF, Ekeberg Ø, Kaasa S. Fatigue and psychiatric morbidity among Hodgkin's disease survivors. J Pain Symptom Manage 2000; 19: 91–9
- Greil R, Holzner B, Kemmler G, Kopp M, Buchowski A, Oberaigner W, et al. Retrospective assessment of quality of life and treatment outcome in patients with Hodgkin's disease from 1969 to 1994. Eur J Cancer 1999; 35: 698–706
- Hjermstad MJ, Fossa SD, Oldervoll L, Holte H, Jacobsen AB, Loge JH. Fatigue in long-term Hodgkin's disease survivors: A follow-up study. J Clin Oncol 2005; 23: 6587–95
- Hjermstad MJ, Oldervoll L, Fossa SD, Holte H, Jacobsen AB, Loge JH. Quality of life in long-term Hodgkin's disease survivors with chronic fatigue. Eur J Cancer 2006; 42: 327–33
- Ruffer JU, Flechtner H, Tralls P, Josting A, Sieber M, Lathan B, et al. Fatigue in long-term survivors of Hodgkin‘s lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG). Eur J Cancer 2003; 39: 2179–86
- Ng AK, Li S, Recklitis C, Neuberg D, Chakrabarti S, Silver B, et al. A comparison between long-term survivors of Hodgkin's disease and their siblings on fatigue level and factors predicting for increased fatigue. Ann Oncol 2005; 16: 1949–55
- Press OW, LeBlanc M, Lichter AS, Grogan TM, Unger JM, Wasserman TH, et al. Phase III randomized intergroup trial of subtotal lymphoid irradiation versus doxorubicin, vinblastine, and subtotal lymphoid irradiation for stage IA to IIA Hodgkin's disease. J Clin Oncol 2001; 19: 4238–44
- Ganz, PA, Moinpour, CM, McCoy, S, Pauler, DK, Press, OW, Fisher, RI. Predictors of vitality (energy/fatigue) in early stage Hodgkin's disease (HD): Results from Southwest Oncology Group (SWOG) Study 9133. J Clin Oncol 2004;22:6546, (Abstract)
- Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol 1999; 161: 182–7
- Herskind C, Bamberg M, Rodemann HP. The role of cytokines in the development of normal-tissue reactions after radiotherapy. Strahlenther Onkol 1998; 174(Suppl 3)12–5
- Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Ann N Y Acad Sci 2001; 933: 222–34
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 1998; 105: 83–107
- Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer 2001; 92(6 Suppl)1684–8
- Bower, JE, Ganz, PA, Tao, ML, Fahey, JL. Mechanisms of radiation-induced fatigue: Proinflammatory cytokines and depression. Paper presented at the California Breast Cancer Research Program Symposium. September, 2003, (Abstract).
- Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage 1993; 8: 196–200
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006; 12: 2759–66
- Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. JNCI Cancer Spectrum 2003; 95: 1165–8
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology 2005; 30: 92–100
- Bower, JE, Ganz, PA, Aziz, N, Olmstead, R, Irwin, MR, Cole, SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain Behav Immun 2006
- Mills, P, Adler, K, Perez, C, Johnson, S, Cohen-Zion, M, Dimsdale, J, et al. Solubel ICAM-1 and fatigue in breast cancer patients in response to chemotherapy. Psychosomatic Medicine 2003;65, (Abstract)
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005; 67: 277–80
- Bower JE. Prevalence and causes of fatigue after cancer treatment: The next generation of research. J Clin Oncol 2005; 23: 8280–2
- Mock V. Evidence-based treatment for cancer-related fatigue. J Natl Cancer Inst Monographs 2004; 32: 112–8
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol 2003; 21: 1660–8
- Helgeson VS, Cohen S, Schulz R, Yasko J. Education and peer discussion group interventions and adjustment to breast cancer. Arch Gen Psychiatry 1999; 56: 340–7
- Helgeson VS, Cohen S, Schulz R, Yasko J. Long-term effects of educational and peer discussion group interventions on adjustment to breast cancer. Health Psychol 2001; 20: 387–92
- Fawzy FI, Cousins N, Fawzy NW, Kemeny ME, Elashoff R, Morton D. A structured psychiatric intervention for cancer patients. I. Changes over time in methods of coping and affective disturbance. Arch Gen Psychiatry 1990; 47: 720–5
- Spiegel D, Bloom JR, Yalom I. Group support for patients with metastatic cancer. A randomized outcome study. Arch Gen Psychiatry 1981; 38: 527–33
- Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: Results of a nursing randomized clinical trial. Oncol Nurs Forum 2002; 29: 949–56
- Jacobsen PB, Meade CD, Stein KD, Chirikos TN, Small BJ, Ruckdeschel JC. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol 2002; 20: 2851–62
- Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, et al. Outcomes from the moving beyond cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol 2005; 23: 6009–18