Abstract
RNA interference (RNAi) induced by small interfering RNA (siRNA) can trigger sequence-specific gene silencing in mammalian cells. It has been proposed that siRNA can be developed as a novel strategy for cancer therapy. However effective delivery of therapeutically active siRNAs into the target tissue/cells in vivo is still a major obstacle for successful application. Oncolytic adenoviral vector mediated RNAi provides the potential advantages of minimizing the harm of normal cells, regenerating siRNAs within the tumor microenvironment and inspiring an additive antitumor outcome through viral oncolysis. Hepatocellular carcinoma (HCC) displays a high resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cell death, partially due to high expression levels of the X-linked Inhibitor-of-Apoptosis protein (XIAP). Here, we utilized an oncolytic adenovirus (ZD55) for expressing short hairpin RNA (shRNA), a precursor of siRNA, to knockdown XIAP. To increase sensitivity of HCC cells to TRAIL, we have used ZD55 to deliver both XIAP-shRNA and TRAIL into HCC cells. The results showed taht the combination of ZD55-XIAP-shRNA and ZD55-TRAIL resulted in significant reduction of XIAP expression and potent antitumor activity both in HCC cells and in animal model with tumor. This pilot study offers a promise of using oncolytic adenovirus to deliver siRNA targeting overexpressed oncogenes and a novel strategy for cancer therapy by regulating the equilibrium between the proapoptotic and antiapoptotic factors.
| Abbreviations | ||
| RNAi | = | RNA interference |
| siRNA | = | small interfering RNA |
| shRNA | = | short hairpin RNA |
| HCC | = | hepatocellular carcinoma |
| TRAIL | = | tumor necrosis factor–related apoptosis–inducing ligand |
| XIAP | = | X-linked inhibitor of apoptosis protein |
RNA interference (RNAi) mediated by small interfering RNA (siRNA) has recently become a powerful technique for cancer gene therapy that is capable of suppressing expression of individual genes with a high degree of specificity Citation[1]. It promises to be an invaluable therapeutic agent that can be employed to silence pathogenic gene products associated with malignant diseases. These siRNA molecules can be introduced into cells directly as synthetic siRNAs or indirectly as precursor long double-stranded RNAs (dsRNAs) or short-hairpin RNAs (shRNAs). Chemically-synthesized siRNAs are quick and easy to obtain, but they have been restricted because of the short half-lives and the high cost for synthesis. Moreover, under certain conditions chemically-synthesized siRNAs can activate the interferon system in mammalian cells, which leads to a shutdown of the synthesis in some proteins and non-specific degradation of cellular mRNAs Citation[2], Citation[3]. To overcome these disadvantages, a set of siRNAs were synthesized in vitro using T7 RNA polymerase instead of chemical synthesis, which lacked the 5′-triphosphate end that is important to avoid the interferon response. However high dose of such kinds of siRNAs still can induce interferon Citation[4]. Instead, plasmid-based vectors expressing shRNA under the polymerase-III promoters (H1 or U6) have been developed. However, plasmid vectors perform low transfection efficiency and unstable expression of shRNAs, when used in vivo Citation[5], Citation[6]. To circumvent these problems, viral vectors carrying shRNA expression cassettes have been developed in an attempt to achieve high delivery efficacy and long-term expression. Several viruses have been constructed as siRNA delivery systems, such as lentivirus, adenoassociated virus (AAV), baculovirus and replication-deficiency adenovirus. These studies have clearly demonstrated that viral vectors expressing siRNA could effectively inhibit gene expression either in vitro cell culture or in vivo local organs Citation[7–9]. However, if this study is applied for therapy of cancer, siRNAs have to be delivered into most of the tumor cells.
As an alternative, tumor selective replicating adenoviruses (also called as oncolytic Ad or CRAds), can overcome the limitations encountered by these siRNA delivery carriers mentioned above. Carette et al. have constructed CRAds expressing shRNA against luciferase and they found this vector could silence up to 70% of the luciferase expression in several cancer cell lines Citation[10]. Recently, Zhang et al. have utilized an oncolytic adenovirus to deliver siRNA targeting mutant K-ras to human cancer cells, resulting in a significantly enhanced antitumor outcome through oncogene knockdown and viral oncolysis Citation[11]. In our study, we have used an oncolytic adenovirus (ZD55) as shRNA carrier to knockdown overexpressed X-linked inhibitor of apoptosis protein (XIAP), a cancer-related pathogenic protein in hepatocellular carcinoma (HCC). ZD55, which can selectively replicate in a variety of tumor cells owing the loss of E1B activity, is similar to the typical viro-therapeutic agent ONYX-015 with E1B 55-kDa gene deletion. However, ZD55 vector system allows incorporating therapeutic genes to construct armed oncolytic adenoviruses. As an effective shRNA deliver system, ZD55 can not only attain significant tumorcidal effect due to tumor specific viral replication, but also restrict shRNA expression within the tumor microenviroment. In this study, we have examined whether oncolytic Ad expressing shRNA could inhibit endogenous overexpressed antiapoptotic gene.
Like many other tumor cells HCC cells display a high expression of inhibitor of apoptosis proteins (IAPs). XIAP is a member of IAP family. This protein is a potent suppressor of apoptosis owing to its ability to bind and inhibit caspases 3, 7 and 9 directly Citation[12]. Overexpression of XIAP has been shown to be associated with high resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cell death in HCC cells Citation[13]. To overcome tumor resistance to TRAIL, we have constructed oncolytic adenoviruses to deliver both of TRAIL and shRNA against XIAP. We investigated their antitumor activity in vitro cell culture and in vivo animal model. Our data showed that significant knockdown of XIAP induced by ZD55-XIAP-shRNA could rapidly promote sensitivity and enhance apoptosis of HCC tumor cells to TRAIL; even ZD55-XIAP-shRNA alone could markedly induce cell death in some HCC cells.
Materials and methods
Cells and cell culture
The normal human lung fibroblast cell line NHLF, normal human liver cell line L-02, and human hepatocellular carcinoma cell lines BEL7404 (with p53 deletion) Citation[14] and SMMC7721 were purchased from the Shanghai Cell Collection (Shanghai, China). Human HCC cell line MHCC97-H with high metastatic potential was provided by Zhongshan Hospital, Fudan University (Shanghai, China). HEK293 (human embryonic kidney cell) was obtained from Microbix Biosystems (Toronto, ON, Canada). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCO-BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO-BRL) at 37°C in a 95% air-5% CO2 humidified incubator.
Animals
Female BALB/c nude mice at 4–5 weeks old were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). All procedures were performed according to institutional guidelines and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Designing and cloning of shRNA directed against XIAP
Plasmid psiRNA-hH1neo (InvivoGen, San Diego, CA, USA) was used to express small hairpin RNA (shRNA) targeting human XIAP. The following hairpin inserts were used, forward, XIAP shRNA: 5′-ACCTC GTGTACCTGCAGACATCAATA TCAAGAG TATTGATGTCTGCAGGTACAC TT -3′, and reverse, 5′-CAAAAA GTGTACCTGCAGACATCAATA CTCTTGA TATTGATGTCTGCAGGTACAC G-3′; scrambled negative shRNA control: 5′- ACCTC GCTTGACTGAACACTTGACTA TCAAGAG TAGTCAAGTGTTCAGTCAAGC TT -3′, and reverse, 5′- CAAAAA GCTTGACTGAACACTTGACTA CTCTTGA TAGTCAAGTGTTCAGTCAAGC G-3′. The insert was cloned into a BbsI site, which is downstream of the H1 promoter, a RNA polymerase III promoter. All the constructions were confirmed by DNA sequencing.
Construction of recombinant viruses
The final shRNA expression cassettes were amplified with forward primer (5′-GGC CTCGAGTTAATCGATACTAGTAA-3′) and reverse primer (5′-GGCACGCGTTAAT TAAGTCTAGAAG-3′) and cloned into the newly modified pZD55 at XhoI/MluI, named as pZD55-shRNA. pZD55 was constructed previously by deleting the E1B 55-KDa gene and introducing a BglII cloning site, we have again modified it, by introducing a multiple cloning site in stead of the single BglII site.
Generation of oncolytic adenoviruses ZD55-XIAP-shRNA and ZD55-shRNA control (ZD55 carrying scrambled negative shRNA control) were carried out according to the protocols of Microbix Biosystems. ZD55-TRAIL, ONYX-015 and wild-type adenovirus (Ad-wt) have been prepared in our lab before Citation[13], Citation[15], Citation[16]. Large-scale purification of adenoviruses was performed by ultracentrifugation with cesium chloride. The titers were determined by plaque assay on HEK293 cells.
Virus progeny assay
To determine virus progeny, tumor or normal cells were infected with ZD55-XIAP-shRNA, ZD55-shRNA control, ONYX-015 or Ad-wt at a multiplicity of infection (MOI) of 5. After 2 h, medium was removed and washed three times with PBS, then added 2 ml fresh medium. Two days post-infection, cells were collected, and virus was released by freeze–thawing for three cycles and centrifuged to collect the supernatant. Virus production was determined by standard plaque assay on HEK293 cells.
Crystal violet staining
Cytotoxicity in HCC cell lines (BEL7404 and MHCC97-H) and normal cell lines (NHLF and L-02) was measured by crystal violet staining as follows. Cells were plated in a 24-well plate and treated with Ad-wt, ONYX-15 or ZD55-XIAP-shRNA at various MOIs. After 5 days, medium was aspirated from cell cultures, and adherent cells were fixed with 4% formaldehyde in PBS for a 10-min period at room temperature. After fixation, cells were washed and incubated for 15 min with 1% crystal violet dissolved in 70% ethanol. Hereafter, cells were washed with water, air-dried, and scanned on a Bio Imagine System scanner.
Western Blot analysis
Total proteins were separated on 8–12% polyacrylamide gels and transferred onto 0.45 µm nitrocellulose in a buffer containing 25 mM Tris-HCl (pH 8.3)/192 mM glycine/20% methanol and blocked with Odyssey blocking buffer (LI-COR; Lincoln, NE) at least 1 h. Membranes were incubated with primary antibodies, detected by the addition of anti-rabbit IR Dye 700 (dilution: 1:3000) or anti-mouse IR Dye 800 (dilution: 1:3000) (Rockland Inc., from Lorne Laboratories, Reading, UK) and fluorescent signal was revealed by using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). The primary antibodies anti-XIAP (dilution: 1:1000) and anti-actin (dilution: 1:1000) were from Santa Cruz Biotechnology.
In vitro cell viability assay
Cells were plated in 96-well plates and treated with various adenoviruses. At the indicated times, cell survival was quantified by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, 0.5 mg/ml) assay.
HCC xenograft tumor in nude mice
HCC xenograft tumor model in nude mice was established as previous description Citation[13]. The BEL7404 cells (2×106) were injected subcutaneously into the lower right flank of female nude mice. When tumors reached 100–200 mm3, mice were divided randomly into five groups (eight mice/group) and were treated by four intratumoral injections of ONYX-015, ZD55-XIAP-shRNA, ZD55-TRAIL plus ONYX-015, or ZD55-TRAIL plus ZD55-XIAP-shRNA at 2.5×108 plague-forming units every 2 days or PBS as a control. The tumor was monitored every 5 days by measuring tumor size using caliper. The tumor volume was calculated by the following formula: V = length×width2×0.5.
For terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) evaluation and immunohistochemistry analysis (shown below), animals were sacrificed on day 7 after treatment. Tumor samples were taken, fixed with formalin, and embedded in paraffin.
TUNEL Assay
In situ cell apoptosis of tumor sections taken on day 7 post-treatment was analyzed by using a commercially available TUNEL kit (Sino-American Biotechnology Company, Luoyang, China), according to the manufacturer's procedures.
Immunohistochemical study
Sections from tumor (on day 7 post-treatment) were incubated with anti-TRAIL (dilution: 1:100) or anti-XIAP (dilution: 1:100) antibodies. The antibody staining signals were amplified by a biotinylated-peroxidase-conjugated streptavidin system (Bio-Genex Laboratories, San Reman, CA, USA). XIAP or TRAIL stain was visualized after reacting with 3, 3′-diaminobenzidine. Hematoxylin was used as a counterstain.
Statistical analysis
The statistical significance of experimental results was calculated by analysis of variance (ANOVA) and student t-test. Data were considered statistically significant at p < 0.05.
Results
Efficient silencing of the XIAP in HCC cells by ZD55-mediated RNAi
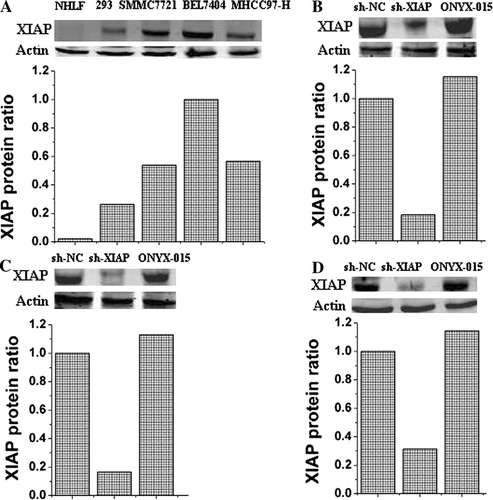
A high level of XIAP protein was found in HCC cell lines (BEL7404, SMMC7721 and MHCC97-H), comparable or higher than the level found in normal cell line (NHLF) or HEK293 cells (A). To evaluate the silencing capacity of XIAP-shRNA, three HCC cell lines (BEL7404, SMMC7721 and MHCC97-H) were treated with ZD55-XIAP-shRNA, ZD55-shRNA control or ONYX-015 as negative controls. Western blot has been performed to detect the XIAP protein levels at 3 days post-transfection. We found that ZD55 mediated RNAi was quite effective and resulted in significant knockdown of the target protein (B,C,D). No XIAP silencing was seen in the presence of the ZD55-shRNA control or ONYX-015. These results suggest that oncolytic adenoviral vectors can be efficient siRNA vehicles in vitro as well as other viruses.
Figure 1. Western blot analysis and quantification of XIAP protein levels by the Odyssey Infrared Imaging System. Relative expression of XIAP was detected in normal cell line (NHLF), HEK293 and HCC cell lines (BEL7404, SMMC7721 and MHCC97-H) (A). Evaluation of siRNA-mediated XIAP knockdown by infecting with ZD55-XIAP-shRNA (sh-XIAP), ZD55-shRNA control (sh-NC), or ONYX-015 was performed in human HCC cell lines BEL7404 (B), SMMC7721 (C) and MHCC97-H (D).
Construction and characterization of oncolytic adenovirus expressing shRNA against XIAP (ZD55-XIAP-shRNA)
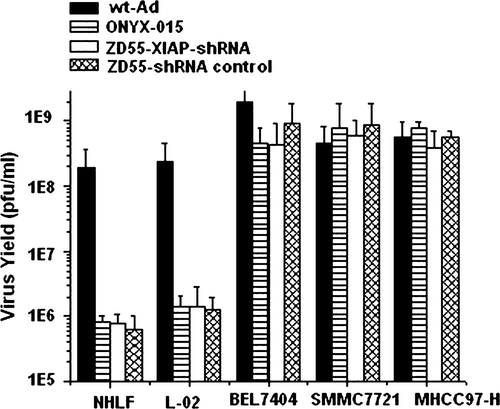
Due to the deletion of E1B 55-kDa from Ad5, ZD55 can selectively replicate in and lyse in a number of tumor cells, which is similar to ONYX-015. In this vector, we have used the human H1 promoter to control transcription of shRNA against XIAP. To determine whether XIAP-shRNA or scrambled control shRNA interfere with the tumor-selective replication ability of ZD55 adenovirus vector, HCC cell lines (BEL7404, SMMC7721 and MHCC97-H) and normal cells (NHLF and L-02) were infected with ZD55-XIAP-shRNA, ZD55-shRNA control, ONYX-015 or wt-Ad. Then, the accumulation of viral particles in culture medium and cell lysate was determined by plaque assay on HEK293 cells. As shown in , ZD55-XIAP-shRNA and ZD55-shRNA control replicated at levels comparable to ONYX-015 and wt-Ad in tumor cells. Similarly to ONYX-015, ZD55-XIAP-shRNA and ZD55-shRNA control did not replicate in normal cells. This data indicated that either shRNA structure or inhibition of XIAP expression did not change viral replication in tumor cells and did not convert normal cells for viral replication.
Figure 2. Selective replication of ZD55 in vitro. Tumor cells (BEL7404, SMMC7721 and MHCC97-H) and normal cells (NHLF and L-02) were infected with ZD55-XIAP-shRNA, ZD55-shRNA control, ONYX-015 or Ad-wt. After 48 h, medium and cells were collected and subjected to three freeze–thaw cycles. The collected supernatants were tested for virus production by standard plaque assay on HEK293 cells.
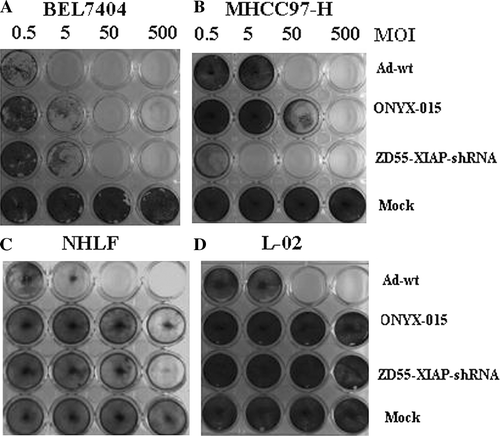
To detect the selective cytotoxity of ZD55-XIAP-shRNA in cells, HCC cells (BEL7404 and MHCC97-H) and normal human lung fibroblasts (NHLF) and normal liver cells (L-02) were infected with Ad-wt, ONYX-015, and ZD55-XIAP-shRNA at various MOIs. Cells were stained with crystal violet 5 days later. In BEL7404 cells, ONYX-015 and ZD55-XIAP-shRNA performed equal cytotoxity, but slightly less than that of Ad-wt. While in MHCC97-H cell, ZD55-XIAP-shRNA rapidly suppressed cell proliferation, which is different from that of Ad-wt and ONYX-015, because of the sensitivity to XIAP-shRNA. Fortunately, significant oncolytic effect was not observed in NHLF and L-02 cells infected with either ZD55-XIAP-shRNA or ONYX-015 ().
Figure 3. The tumor selective cytotoxic effect of ZD55-XIAP-shRNA. Tumor cell lines BEL7404 (A), MHCC97-H (B) and normal cell lines NHLF (C) and L-02 (D) were infected with Ad-wt, ONYX-015 or ZD55-XIAP-shRNA at the indicated MOIs. Five days later, cells were stained with crystal violet.
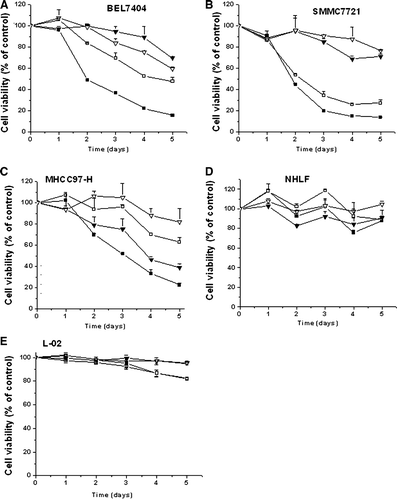
To further evaluate the kinetics of cytotoxicity induced by these viruses, three HCC cell lines (BEL7404, SMMC7721 and MHCC97-H) and two normal cell lines (NHLF and L-02) were plated in 96-well plates and infected with viruses, and an MTT assay was performed. The data showed that the cytotoxic effect of ZD55-XIAP-shRNA on the BEL7404 and SMMC7721 cell lines was similar to the effect of ONYX-015, but more apparent in MHCC97-H cell line, and slight cytopathic effect on normal cells ().
Figure 4. Synergistic antitumor activity of ZD55-XIAP-shRNA and ZD55-TRAIL in human HCC cells. Tumor cells BEL7404 (A), SMMC7721 (B) and MHCC97-H (C) and normal cells NHLF (D) and L-02 (E) were infected with ZD55-XIAP-shRNA (▾), ONYX-015 (7), ZD55-TRAIL plus ZD55-XIAP-shRNA (▪) and ZD55-TRAIL plus ONYX-015 (7). Cell viability was determined by MTT assay. Data was presented as means±SD as three independents (p < 0.01).
Consequently, virus progeny assay indicated that shRNA would not interfere with the tumor-selective replication characteristic of ZD55, and both the data of MTT assay and crystal violet staining showed that siRNA-mediated diminished expression of XIAP significantly enhanced the cytotoxic activity of the oncolytic viral vector in MHCC97-H cells, but not in BEL7404, SMMC7721, NHLF and L-02 cells. These observations suggest that XIAP may play a different role in regulating apoptosis of different HCC cells and ZD55-XIAP-shRNA is not harmful to normal cells. Moreover, the results also indicated that ZD55 could be a rational tumor-specific replicative adenovirus for shRNA delivery and shRNA expression would not interfere with the virus replication and oncolytic activity.
Inhibition of XIAP enhances sensitivity to TRAIL of HCC cells
Our previous study has shown that HCC cells overexpressing XIAP were more resistant to apoptosis induced by TRAIL Citation[13]. A possible role of downstream inhibitors of apoptosis such as XIAP that bind to FADD or other proteins in caspase pathway has been postulated Citation[17–19].
To investigate the effects of XIAP-shRNA on the sensitivity of HCC cells to TRAIL, cells were infected either with ONYX-015, ZD55-XIAP-shRNA, ONYX-015 plus ZD55-TRAIL, or with ZD55-XIAP-shRNA plus ZD-TRAIL. Cell viability was assessed by MTT assay. A significant decrease in cell viability was observed in HCC cells treated with a combination of ZD-XIAP-shRNA and ZD55-TRAIL, compared with ZD55-XIAP-shRNA alone, ONYX-015 alone, or combination of ZD55-XIAP-shRNA plus ONYX-015 (p < 0.01) (A,B,C).
In addition, all these viruses did not have big impact on the growth of normal cells (D,E), although ZD55-TRAIL plus ONYX-015 or ZD55-TRAIL plus ZD55-XIAP-shRNA group showed slight toxicity to normal liver cells (L-02) (E).
Synergistic antitumor activity of ZD55-XIAP-shRNA and ZD55-TRAIL in nude mice
To further evaluate the synergistic activity of ZD55-XIAP-shRNA combined with ZD55-TRAIL in suppressing tumor growth in vivo, we established a tumor model in nude mice bearing human HCC xenografts. Tumor growth of the group treated with ZD55-XIAP-shRNA plus ZD55-TRAIL was obviously inhibited, as compared with the animals treated with ZD55-XIAP-shRNA, ONYX-015 or PBS (p < 0.01). However compared with the group treated with ZD55-TRAIL plus ONYX-015, the synergistic antitumor activity of ZD55-XIAP-shRNA and ZD55-TRAIL was more prominent at the early time points than during the later stages of the experiment ().
Figure 5. Antitumor efficacy of ZD55-XIAP-shRNA and/or ZD55-TRAIL in BEL7404 xenograft tumor. When the tumor reached 100–200 mm3, animals were treated with an intratumoral injection of 1×109 pfu per animal of ZD55-XIAP-shRNA (▾), ONYX-015 (7), ZD55-TRAIL plus ZD55-XIAP-shRNA (▪), ZD55-TRAIL plus ONYX-015 (7), or PBS (:) as a control (the first injection indicated by arrow). The tumor size was measured and tumor volume was calculated as: length×width2×0.5. Data are expressed as means of tumor volume±SD (n = 8).
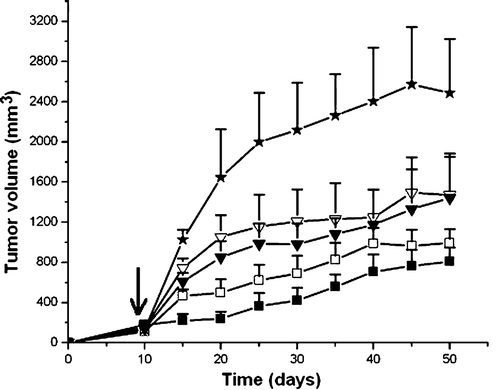
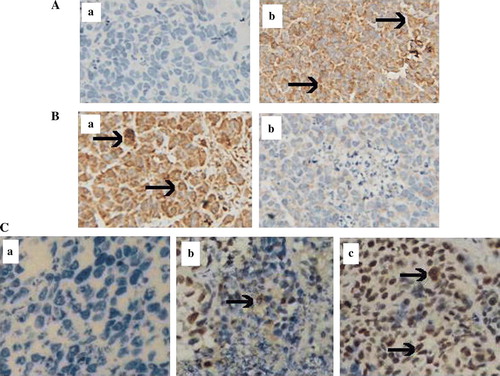
To understand the mechanism of tumor inhibition, tumor sections were analyzed for apoptosis by TUNEL assay. As shown in C, tumors from the mice injected with ZD55-XIAP-shRNA plus ZD55-TRAIL demonstrated extensive apoptosis, compared with the group treated with ZD55-TRAIL plus ONYX-015 or PBS. Moreover, strong TRAIL expression was observed in tumor section after treatment of ZD55-XIAP-shRNA plus ZD55-TRAIL (A). High level of XIAP expression was observed in the tumor section from animal receiving PBS and administration of ZD55-XIAP-shRNA resulted in dramatic reduction of XIAP expression in tumor sections on day 7 after therapy (B).
Figure 6. Immunohistochemical and apoptotic study in vivo 7 day post-treatment with viruses. A immunohistochemistry was performed in the tumor sections by reaction with the primary antibody against TRAIL, group b treated with ZD55-TRAIL plus ZD55-XIAP-shRNA displayed much higher TRAIL expression than group a treated with PBS as control. B XIAP expression level in vivo, group b treated with ZD55-TRAIL plus ZD55-XIAP-shRNA indicated much lower staining than group a (PBS). C TUNEL assay was performed to detect apoptosis in the tumor section: group c (ZD55-TRAIL plus ZD55-XIAP-shRNA) showed extensive staining than control group a (PBS) and group b (ZD55-TRAIL plus ONYX-015). (Original magnification×400, arrows denote positive staining).
Discussion
RNAi is a process of sequence-specific post-transcriptional gene silencing mediated by double-stranded RNA. Compared with conventional single-stranded antisense oligonucleotides (ASO) technology, RNAi is more efficient at silencing the corresponding gene expression. Although some proteins, under certain circumstances, can block function or down-regulate expression of other proteins, such as Smac can inhibit XIAP Citation[13], this is a complex process mediated by a group of proteins and only applicable in a few instances, while RNAi can specifically knockdown any target gene flexibly.
At present, the main obstacle to the development of therapeutic agents using RNAi technology is a suitable delivery method. Although non-viral-based siRNA vectors are regarded as the safest delivery systems, they still constitute an essential challenge in therapeutics, limited by their poor ability to escape from the endosomal compartment and to translocate DNA into the nucleus. Another important consideration for siRNA-mediated inhibition of gene expression is whether the observed effects are free from potential IFN responses, as lipid delivery of synthetic siRNAs can reportedly induce immune activation in vivo Citation[20], Citation[21]. In contrast, viral vectors have been used to deliver siRNA widely, and these methods tend to exhibit several advantages over non-viral vectors, such as higher transduction efficacy and more stable gene silencing. However, most of the viral vectors used for siRNA delivery are non-replicative viruses. As an alternative, tumor specific replicating adenovirus can be applied to overcome these limitations. Previous data have showed that shRNAs expressed from an oncolytic adenovirous specifically silenced expression of the firefly luciferase gene in cancer cells. Although silencing efficiency, only was partial, ranging from 40 to 70% knockdown Citation[10].
In our study, we have introduced an oncolytic adenovirus (ZD55) for shRNA delivery to knockdown XIAP in HCC. Our data indicated that ZD55 could be successfully used as a novel tool for XIAP-shRNA delivery in cancer gene therapy. In order to avoid the interferon induced tumor cell death by the shRNA structure, we have tested the cytotoxicity of the scrambled ZD55-shRNA and ONYX-015 in several HCC cell lines, particularly in Huh-7 cells regarded as a sensitive indicator of secreted IFN Citation[22]. Our results showed that there was no difference on tumor cell toxicity between the scrambled ZD55-shRNA and ONYX-015 group (Data not shown). It suggests that shRNA structure does not contribute to the cytotoxic effect of these HCC cells.
ZD55 was constructed based on adenovirus serotype 5 (Ad5), with E1B 55-KDa gene deletion, which is similar to the viral therapeutic agent ONYX-015. Regarding to the oncolytic mechanism of this virus, initial studies indicated that the E1B 55-KDa depleted adenovirus selectively target p53-deficient cells, while approximately half of all human tumors contain defects in the p53 gene Citation[23], Citation[24]. However, subsequent data showed that p53 pathway or late viral RNA export, rather than p53 inactivation, determine ONYX-015 tumor selectivity Citation[25], Citation[26]. Moreover, other studies have determined that actual specificity of viral replication of ONXY-015 is based on the timing of viral replication in tumor cells Citation[27], Citation[28]. Thus, the proposed model of ONYX-015 action is still unclear. But empiric efficacy in tumor treatment has been demonstrated Citation[29]. In addition, our previous work has proved that ZD55 can selectively replicate in HeLa, Bcap37, HCT116, HT-29, SW620 Citation[30]. And in our present study, virus progeny assay has clearly addressed the specific virus replication in BEL7404, SMMC7721 and MHCC97-H ().
Although ONYX-015 has been used extensively in cancer therapy clinical trials, it has not been proven to be very efficient Citation[31], Citation[32]. To improve the therapeutic efficiency, we combined this virotherapeutic agent with RNAi technology and conventional gene therapy method. In this study, ZD55-mediated siRNA can efficiently silence the expression of XIAP in vitro, leading to directly induce cell death in MHCC97-H cell line and increase the sensitivity of all three HCC cell lines to ZD55-TRAIL mediated apoptosis, but no apparent impacts on normal cells (NHLF and L-02). XIAP is one of the most potent caspase inhibitors in the IAP family and inhibits apoptotic cell death predominantly by preventing activation of initiator caspase 9 as well as effector caspases 3 and 7 Citation[33], Citation[34]. Notably, overexpression of XIAP is capable of preventing apoptosis induced by a variety of triggers in vitro and has been shown to inhibit cell death in several models in vivo Citation[35–37]. Previous study has showed that suppression of XIAP by overexpression of Smac did not induce activation of caspases and result in cell apoptosis in the absence of apoptotic stimuli Citation[38]. In our investigation, we observed that ZD-XIAP-shRNA alone could strongly trigger cell death in MHCC97-H cell line, probably due to the late stages of viral replication that could induce cell death Citation[39]. Thus ZD55 itself may be thought as an apoptotic trigger. However, the similar results did not exist in other HCC cells as might indicate that XIAP plays a different role in different HCC cell lines in regulating apoptotic pathway.
To further prove the synergistic efficacy, our in vivo data showed that ZD55-XIAP-shRNA could promote antitumor activity of ZD55-TRAIL in HCC xenograft tumors. However the effect was not so adequate as compared with in vitro experiments, particularly at late stage of the in vivo experiment. The phenomena may suggest that conventional oncolytic adenoviral vectors are not so efficient for long-term siRNA delivery. The reason may be probably due to elimination of oncolytic adenovirus by host or expression of the noncoding adenovirus VA RNAI and VA RNAII that have the capacity to suppress RNAi at late times of infection as reported recently Citation[40].
In conclusion, our ZD55 vector has both the oncolytic ability and siRNA delivery capability. ZD55-XIAP-shRNA could knockdown XIAP expression and enhance the antitumor activity of ZD55-TRAIL both in vitro and in vivo. However the findings suggested that these oncolytic adenoviruses need to be further modified, such as deleting VA RNAI and VA RNAII, in order to provide a more efficient oncolytic vehicle for siRNA delivery in cancer therapy.
Acknowledgements
This work was supported by the 973 Project (No.2004CB518804), the 863 project (No.20060102Z1107) and Zhejiang Provincial Grant (No.2006C23006). The authors thank Dr. Lanying Sun, Wei Zhang and Yanling Ren for help with the cell culture and animal experiment.
References
- Scherer L, Rossi JJ. RNAi applications in mammalian cells. Biotechniques 2004; 36: 557–61
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 2001; 15: 188–200
- Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat Biotechnol 2004; 22: 321–5
- Donze O, Picard D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res 2002; 30: e46
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–3
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester Wc, et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 5515–20
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003; 33: 401–6
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 2003; 9: 1539–44
- Nicholson LJ, Philippe M, Paine AJ, Mann DA, Dolphin CT. RNA interference mediated in human primary cells via recombinant baculoviral vectors. Mol Ther 2005; 11: 638–44
- Carette JE, Overmeer RM, Schagen FH, Alemany R, Barski OA, Gerritsen WR, et al. Conditionally replicating adenoviruses expressing short hairpin RNAs silence the expression of a target gene in cancer cells. Cancer Res 2004; 64: 2663–7
- Zhang YA, Nemunaitis J, Samuel SK, Chen P, Shen Y, Tong AW. Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Res 2006; 66: 9736–43
- Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol 2004; 24: 7003–14
- Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, et al. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology 2004; 39: 1371–81
- Zhao H, Xu Y. Mad-overexpression down regulates the maliganant growth and p53 mediated apoptosis in human hepatocellular carcinoma BEL7404 cells. Cell Res 1999; 9: 51–9
- Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res 2003; 13: 481–9
- Liu XY, Qiu SB, Zou WG, Pei ZF, Gu JF, Luo CX, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol Ther 2005; 11: 531–41
- Chawla-Sarkar M, Leaman DW, Jacobs BS, Boden EC. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol 2002; 169: 847–55
- Dong ZX, Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis inducing ligand (TRAIL) receptor and Flice inhibitory protein expression to TRAIL induced apoptosis of melanoma cells. Cancer Res 1999; 59: 2747–53
- Zhang XD, Zhang XY, Gray CP, Nguyen T, Hersey P. Tumor necrosis factor related apoptosis inducing ligand induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria. Cancer Res 2001; 61: 7339–48
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet 2003; 34: 263–4
- Sioud M, Sorensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun 2003; 312: 1220–5
- Vrolijk JM, Kaul A, Hansen BE, Lohmann V, Haagmans BL, Schalm SW, et al. A replicon-based bioassay for the measurement of interferons in patients with chronic hepatitis C. J Virol Methods 2003; 110: 201–9
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumour cells. Science 1996; 274: 373–6
- Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res 1999; 59: 410–3
- Ries SJ, Brandts CH, Chung AS, Biederer CH, Hann BC, Lipner EM, et al. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl 1520 (ONYX-015). Nat Med 2000; 6: 1128–33
- O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONXY-015 tumor selectivity. Cancer cell 2004; 6: 611–23
- Goodrum FD, Ornelles DA. p53 status does not determine outcome of E1B 55 kilo-dalton mutant adenovirus lytic infection. J Virol 1998; 72: 9479–90
- Turnell A, Grand R, Gallimore P. The replicative capacities of large E1B-null group A and group C adenovirus are independent of host cell p53 status. J Virol 1999; 73: 2074–83
- Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol 2001; 19: 289–98
- Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther 2005; 16: 845–58
- Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: What have we learned?. Gene Ther 2001; 8: 89–98
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): Results of phase I and II trials. Expert Opin Biol Ther 2001; 1: 525–38
- Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis 2001; 6: 253–61
- Salvesen GS, Duckett CS. IAP proteins: Blocking the road to death's door. Nat Rev Mol Cell Biol 2002; 3: 401–10
- Xu D, Bureau Y, McIntyre DC, Nicholson DW, Liston P, Zhu Y, et al. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J Neurosci 1999; 19: 5026–33
- Kugler S, Straten G, Kreppel F, Isenmann S, Liston P, Bahr M. The X-linked inhibitor of apoptosis (XIAP) prevents cell death in axotomized CNS neurons in vivo. Cell Death Differ 2000; 7: 815–24
- Perrelet D, Ferri A, Liston P, Muzzin P, Korneluk RG, Kato AC. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat Cell Biol 2002; 4: 175–9
- Ng CP, Bonavida B. X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: Sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO). Mol Cancer Ther 2002; 1: 1051–8
- White E. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 2001; 20: 7836–46
- Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B, Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol 2005; 79: 9556–65