Abstract
Introduction. Cancer rehabilitation programs mainly involve endurance training, and little attention is paid to strength training. Cancer survivors are generally advised to train at much lower workloads than the standard guidelines for strength training suggest. The purpose of this study is to evaluate the effectiveness of an 18-week high-intensity strength training program in cancer survivors. Methods. Fifty-seven patients (age 24 to 73 years) who had received chemotherapy for lymphomas, breast, gynecologic, testicular, or colorectal cancer completed the program. Outcome measures were changes in muscular strength (one-repetition maximum), cardiopulmonary function (VO2 max), maximal short exercise capacity (MSEC), body composition and health-related quality of life (HRQOL) between baseline and follow-up. Discussion. The high-intensity strength training was well tolerated by all patients. Significant improvements in muscle strength were found, with effect sizes varying from 1.32 to 2.68. VO2 max increased significantly by 10% in men and by 13% in women. Different functional scales of HRQOL improved (p < 0.01), with effect sizes varying from 0.47 to 0.82. Muscle strength correlated significantly with physical functioning before and after the training program. Conclusion. We conclude that a supervised, high-intensity strength training program seems to be an effective means to improve muscle strength, cardiopulmonary function, and HRQOL and should be incorporated in cancer rehabilitation programs. Further randomized trials are needed to confirm the results.
Cancer and its treatment are often associated with adverse physical side-effects including muscular atrophy, decreased muscle strength and reduced aerobic capacity Citation[1]. These side-effects may contribute to the development of cancer-related fatigue Citation[2]. About 70% of cancer patients report fatigue complaints during chemotherapy and/or radiotherapy Citation[3–5]. Even years after the treatment, fatigue is still a problem for up to 30% of cancer survivors and has a great impact on the patient's quality of life Citation[6], Citation[7].
Until recently, most physicians recommended rest or a reduction in the amount of physical activity as treatment for fatigue Citation[8]. This created a physiological paradox because inactivity induces muscle catabolism, causing further deconditioning, which incites even more fatigue Citation[2]. Recently, several reviews of the literature examined the effect of physical activity in cancer survivors Citation[1], Citation[9–13]. Overall, most studies demonstrated that physical training programs had beneficial effects on cancer patients’ physical or psychosocial capacity. For instance, physical activity in cancer survivors has been shown to improve aerobic capacity, muscle strength, body composition, and quality of life and to reduce fatigue Citation[14–23]. Unfortunately, most training studies in cancer patients were performed with a limited number of subjects (n < 40) and duration of less than 12 weeks. Studies mainly included breast cancer patients or patients who had already participated in physical activity before treatment. Another aspect was that most studies used aerobic exercises such as walking or stationary cycling Citation[3], Citation[16], Citation[24]. This is somewhat surprising since muscle atrophy is a common problem in cancer patients Citation[25], which warrants strength training as the physical training program of choice.
Muscle atrophy results from a sedentary lifestyle and prolonged bed rest, which is worsened in cancer patients by tumor factors and the side-effects of medication (e.g. glucocorticoids or chemotherapeutic agents) on the skeletal muscle structure and function Citation[2], Citation[12], Citation[26]. Skeletal muscle, however, has shown great adaptability with appropriate training stimuli even in cases of severe muscle atrophy and fatigue Citation[2]. Progressive strength training has been shown to increase lean body mass, muscle protein mass and contractile force, and improves physical function in healthy, young and elderly subjects Citation[27]. Considerable evidence now suggests that the ability to perform physical tasks in daily life is determined by a threshold level of muscular strength Citation[28]. As a consequence, strength training in cancer patients would seem to be a potent physiological intervention for regaining lost muscles and improving muscle quality and as a result improving the overall quality of life Citation[10].
Only three studies have investigated strength training programs in cancer survivors Citation[20], Citation[21], Citation[29]. Two of these studies employed strength training during treatment while only one study used strength training after treatment. This latter study involved a selected subgroup of prostate cancer patients receiving androgen deprivation therapy Citation[20], and it is therefore questionable whether these results can be extrapolated to the more general population of cancer patients treated with chemotherapy. Since research regarding strength training in cancer survivors is limited, recommendations about optimal intensity, frequency, and duration of strength training are lacking. The American College of Sports Medicine (ACSM) proposes rather low to moderate exercise intensities Citation[30] if compared with a recent publication about the fundamentals of resistance training Citation[31].
The aims of the study were (1) to obtain more insight in the physical capacity and quality of life status after cancer treatment; (2) to assess the effects of a strength training programme on muscle strength, cardiopulmonary functioning and quality of life; (3) to assess the correlation between muscle strength and outcomes of HRQOL.
Material and methods
Patients
The study was conducted in the community hospital Máxima Medical Centre. The project was approved by the Medical Ethics Committee of the Máxima Medical Centre, and informed consent was obtained from all patients. From 2002 onwards, rehabilitation using the experimental training program was implemented as standard medical care after chemotherapy. Medical oncologists recruited all patients treated with chemotherapy with a curative intention. These patients participated in an intake procedure including medical examination and cardiopulmonary exercise testing. Patients were excluded if they were not able to perform basic skills like sitting or lying down or had cognitive disorders or severe emotional instability or suffered from other serious diseases that might hamper physical performance capacity (e.g. heart failure, COPD, neurological disorders).
Seventy-three consecutive patients were initially referred by oncologists to the rehabilitation program. After the intake procedure by a sports physician, five patients (mean age 53 years) were excluded because of serious co-morbidity. During the training period, six patients (mean age 47 years) dropped out because of cancer recurrence, and five patients (mean age 45 years) dropped out because of family (e.g. divorce, disease of the spouse) and work circumstances. Therefore, 57 patients (13 men, 44 women) completed the entire program. The characteristics of these patients are shown in .
Table I. Patients’ characteristics at time of inclusion.
Training intervention
To counteract bias resulting from spontaneous recovery after chemotherapy, training started no earlier than 6 weeks after completing chemotherapy. The training program consisted of high-intensity strength and interval training for 18 weeks. The patients trained in groups of six to eight persons on specialised strength training equipment and on bicycle ergometers under the supervision of physiotherapists. During the first 12 weeks, the patients trained twice a week under the supervision of a physiotherapist. For the last six weeks, the patients trained once a week under supervision.
High-intensity strength training
The strength program consisted of six exercises targeting the large muscle groups as follows: 1) vertical row (focusing on longissimus, biceps brachii, rhomboideus); 2) leg press (quadriceps, glutei, gastrocnemius); 3) bench press (pectoralis major, triceps); 4) pull over (pectoralis, triceps brachii, deltoideus, trapezius); 5) abdominal crunch (rectus abdominis); 6) lunge (quadriceps, glutei, hamstrings). Firstly, strength exercises were performed at 65% to 80% of one-repetition maximum (1-RM) and consisted of two sets of 10 repetitions. After the twelfth week, the emphasis shifted from muscle strength to muscle endurance involving training with less resistance (35–40% of 1-RM) but more (20) repetitions. Every 4 weeks the training progression was evaluated, and the training result was adjusted by means of a 1-RM test.
Interval training
The interval training consisted of cycling two times 8 minutes, before and after the strength program. In the first 8 weeks, those 8 min consisted of alternating 30 s at 65% of the maximal workload extracted from the steep ramp test (MSEC) and 60 s at 30%. From week nine those 8 min consisted of alternating 30 s at 65% and 30 s at 30% of the MSEC.
Outcome measures
Cardiopulmonary function
The safety of the program was guaranteed by an extensive intake procedure. Before the start of the program, each patient's cardiopulmonary and muscular limitations were determined by consultation with a sports physician and a VO2 max test. The VO2 max test was performed on a cycle ergometer (Corival, Lode, the Netherlands). Expired gases were collected and analyzed breath by breath for O2, CO2 and volume. ECG was continuously monitored. This maximal exercise test was done before and after the training program according to the standard protocol Citation[32]. In addition, VO2 max testing was used as a diagnostic tool to assess potential cardiopulmonary limitations (e.g. blood pressure related) caused by cardiotoxic (e.g. anthracyclins) or pulmotoxic (e.g. bleomycin) medications, or radiation therapy to the breast Citation[33–35].
Body composition
Height and weight were measured and the body mass index (BMI) calculated before and after the training program. Skinfolds at biceps, triceps, subscapular and suprailiac were measured Citation[36]. Percentage body fat was determined from body weight and the skinfold measurements using the equation of Durnin and Womersley Citation[36].
Muscle strength
One-repetition maximum (1-RM) is the maximum amount of weight that can be lifted once. Predicted 1-RM values were estimated from the Brzycki equation to evaluate upper and lower body strength potential Citation[37], Citation[38]. 1-RM is stated in kilograms in proportion to body weight. Muscle groups were tested with the strength equipment that was also used for the training. In order to adapt the training intensity and to gain more insight into the training response, the test was carried out five times (weeks 1, 5, 9, 13 and 18).
Maximal short exercise capacity (MSEC)
A steep ramp test was performed to determine the maximal short exercise capacity. This test was developed for heart rehabilitation Citation[39]. After 30 s of cycling at 25 W, the load was increased by 25 W every 10 s until exhaustion. The patient was instructed to cycle with a pedal frequency between 70 and 80 rpm. The test ended when the pedal frequency fell below 60 rpm. This test was performed at the start of the program and at weeks 5, 9, 13, and 18 to assess the interval training.
Health-related quality of life (HRQOL)
The European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire C30 (EORTC QLQ-C30) was developed to assess the HRQOL of cancer patients. The EORTC QLQ-C30 encompasses 30 items divided into five functional scales (physical, role, cognitive, emotional, and social functioning), three symptom scales (fatigue, nausea, and pain) and six individual items. It also includes two questions about the overall quality of life.
Statistical analyses
Sample size calculation was based on the standard deviations of the changes in muscular strength observed in the first ten subjects recruited in this investigation. It was determined that a minimum of 43 subjects was required to detect a 0.20 1-RM/kg body weight difference, with power and significance set at 90% and 5% (two-tailed), respectively. To compensate for dropouts based on cancer recurrence, extra patients were enrolled. Then χ2 tests for categorical data and independent-samples t-tests for continuous data were used to examine group differences in gender, age, cancer diagnosis, time between completion of treatment and start of training, initial muscle strength, and cardiopulmonary function between dropouts and those who stayed in the programme.
First, analysis of variance (ANOVA) was used to assess the differences in changes in outcome measures between male and female subjects. Since there were no significant differences between men and women, all the patients were analyzed together by two-tailed paired t-tests between the test moments. Effect sizes (ES) were calculated as mean increase divided by the initial standard deviation, to provide an objective and standardized measure of the magnitude of the observed effect. The Pearson correlation coefficient was used to quantify the relation between muscle strength and HRQOL before and after the training program. It was also used to quantify the relation between time span from last treatment and start moment of training and initial muscle strength. This correlation was quantified to assess the effect of spontaneous recovery on muscle strength. Test results were considered significant for p-values < 0.05. All analyses were performed using the statistics program SPSS (version 13.0).
Results
Tolerance of the exercise intervention
Although the training intensity was high, the program was well tolerated by all patients. Six patients dropped out because of cancer recurrence or metastatis, and five patients dropped out because of other reasons (personal (2), not interested anymore, disease of the spouse, malaise). None of the patients dropped out because the program was too intense. The total dropout rate was 16%. When cancer-related reasons were not taken into account, the dropout rate was 7%. The χ2 tests and t-tests revealed no significant differences between the dropouts and the patients who finished the program with respect to gender, age, cancer diagnosis, time between completion of treatment and start of training, cardiopulmonary function, and initial muscle strength. In an opinion poll about the benefit of the program, patients gave an average score of 8.9 on a scale from 1 (poor) to 10 (optimal).
Effects on cardiopulmonary function
Data of the effects of training on cardiopulmonary function are shown in . Before training, the male patient's average VO2 max was 30.7 ml/min/kg. After 18 weeks of training, this increased by 10% (p < 0.01). The mean VO2 max in female patients was 24.2 ml/min/kg, which increased by 13% (p < 0.01). The oxygen consumption at the ventilatory threshold (VO2 VT) increased by 23% in male patients and 13% in female patients (p < 0.01). Both the respiratory quotient (RQ ≥ 1.16) and the ratio maximal heart rate/predicted maximal heart rate (220 beats per minute–age) were high before and after training, indicating that all patients performed until maximum exhaustion in both tests.
Table II. Physiological effects of training.
Effects on body composition
Results of changes in body composition are presented in . Only in male patients were there significant changes. Their weight and BMI increased slightly by 2% after training.
Effects on muscle strength
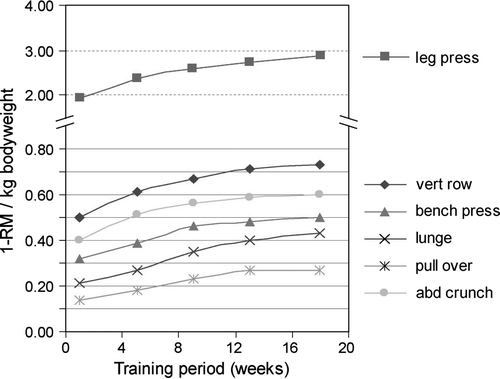
lists the 1-RM test results for all strength exercises before and after training. After training, all 1-RM increased significantly, with effect sizes varying from 1.32 to 2.68, which are considered large effects. shows the results of all measurement time points, before during and after the training program. In all of the strength exercises, there is a substantial and significant improvement following a roughly identical pattern. The largest improvement in muscle strength is seen in the first 8 to 12 weeks of training. The lunge (+105%) and pull over (+93%) show the greatest increases.
Table III. Effects of training on muscle strength.
Effects on HRQOL
Changes in EORTC QLQ-C30 scores are listed in . All function scales, except cognitive functioning, show a significant improvement after training. Physical functioning increased by 17% with an ES of 0.54 (p < 0.01). From the symptom scales and individual items, only fatigue is presented in since the other symptoms are not associated with physical training effects but with the elapsed period of time after chemotherapy and show no significant changes. Fatigue diminished significantly after training by 50% with an ES of −0.83.
Table IV. Effects of training on quality of life: results from EORTC QLQ-C30.
Table V. Correlations between muscle strength and HRQOL.
Association between muscle strength and HRQOL
shows Pearson correlations between muscle strength and physical functioning before and after training. Before training there was a strong association (from 0.56 to 0.75; p < 0.01) between all strength exercises and the physical functioning scale from HRQOL. Mean changes in muscle strength of all exercises correlated with changes in physical functioning (r = 0.43; p < 0.01).
Association between time span from last treatment to start moment of training and initial muscle strength
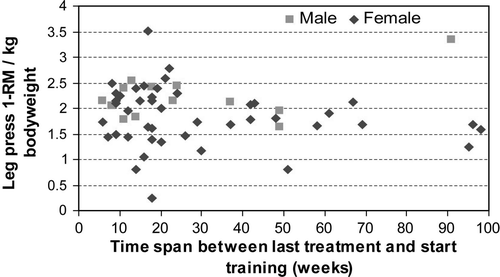
No significant correlations were found between 1) time span from last treatment to start moment of training (mean = 28 weeks, range from 6 weeks to 98 weeks) and 2) initial 1-RM per kg body weight for all strength exercises. depicts the association between this time span and the initial 1-RM for leg press.
Discussion
In the present study we characterized the physiological profile of a heterogeneous group of cancer patients (unselected with respect to referral) after treatment with chemotherapy and the effects of a high-intensity strength training program. Patients were characterized by low strength, a high body fat mass, and a low to normal aerobic capacity (VO2 max). High-intensity strength training as employed in the present study produced significant improvements not only in muscle strength but also in maximal oxygen consumption, maximal workload, and HRQOL. We also found that muscle strength correlated significantly with HRQOL outcomes. The training program proved effective in this heterogeneous group of cancer patients.
Tolerance of the high-intensity program
None of the patients dropped out because of overloading or injuries. The adherence rate was 92%, which is far above average for quality of life interventions. There are several explanations for this high adherence rate. Briefly, the extensive intake procedure by a sports physician, the supervision by physiotherapists during training, and the individualization of training loads based upon appropriate tests all contributed to the safety and efficiency of the program. Special attention was indicated for breast cancer patients since axillary dissection in combination with the fibrosing effect of adjuvant radiotherapy impaired their shoulder function Citation[40], Citation[41].
Changes in cardiopulmonary function
Maximal oxygen consumption, maximal workload, and the oxygen consumption at the ventilatory threshold improved significantly after the training program. The increase of 13% in VO2 max in male patients and 14% in female patients in the present study is in line with other training intervention studies in cancer patients that use aerobic exercise Citation[16–19].
Changes in muscle strength
The present study shows significant improvements in muscle strength after training. The largest increase in muscle strength was observed in the first 8 to 12 weeks of training. These are similar to observations in healthy subjects and reflect the fact that the initial increase in muscular strength can be explained by an improved neuromuscular adaptation. The lunge and pull over exercises show the greatest increase. These exercises are the ones in which substantial progress can be made by improved coordination along with an increase in strength.
Research regarding strength training in cancer survivors is limited. Despite this, all three studies that applied strength training showed advantageous effects on fatigue levels, quality of life, and muscle strength Citation[20], Citation[21], Citation[29]. Segal et al. randomly assigned 82 men (mean age 68 years) with prostate cancer receiving androgen deprivation therapy to a strength training program and 73 men to a waiting list control group. They concluded that a 12-week strength training program (3 times per week with 2 sets of 8–12 repetitions at 60–70% of 1-RM) resulted in a reduction of fatigue and improvement in both quality of life and muscular fitness Citation[20]. These results in prostate cancer patients are in line with our results in a broader population of cancer patients after chemotherapy. Cunningham et al. used strength training in 30 patients undergoing marrow transplantation for acute leukemia. However, the type and intensity of strength training and the outcome parameters for muscle strength were not specified, making comparison with our study difficult Citation[29]. Adamsen et al. examined the effect of high-intensity strength training in 23 cancer patients undergoing chemotherapy. In this training program, three series of 5–8 repetitions at 85–95% of 1-RM involving the large muscle groups were performed. The increase in muscle strength was 33%, and the aerobic capacity increased by 16% Citation[21]. In accordance with our study, this high-intensity strength training program was well tolerated and effective.
On the other hand, the American College of Sports Medicine (ACSM) proposes exercise intensities of 50% of 1-RM with 2–3 sets of 3–5 repetitions building to 10–12 repetitions Citation[30]. From a physiological point of view, these guidelines seems to be too low for an optimal training effect Citation[31], Citation[42]. A possible reason for why these guidelines are rather conservative is an unwillingness to expose these patients to risks, like muscle and joint injuries at high intensities. Also, since cancer patients are undergoing intense psychological and physical stress, they perhaps should be treated “gently” and hence only do low intensity exercise. These reasons are logical because in 2003, when the guidelines were formulated, research about strength training was very limited. It has been shown in healthy subjects that improvements from a strength training program are more effective when heavier loads (higher intensities) are used. Substantial gains in maximal strength and the subsequent hypertrophy can only be achieved when the maximal number of motor units is recruited, warranting high training loads Citation[31]. In addition, other tissues such as bone also respond more favorably to such heavy loading. This is clinically important since in postmenopausal breast cancer survivors, the bone mineral density is lower than normal, and it has been shown that strength training is beneficial in preventing further bone loss Citation[43].
HRQOL and clinical relevance
Although the benefits of strength training have been recognized in healthy subjects, its value for rehabilitation is relatively unexplored. In cancer rehabilitation, an important goal is to counteract the side-effects of the disease and its treatment and consequently improve the quality of life. In this study we found that strength training has beneficial effects on different aspects of quality of life and reduces fatigue. Muscle strength was strongly related to physical functioning before treatment, and changes in muscle strength were correlated with changes in physical functioning. Several biopsychosocial mechanisms may explain the quality of life improvements in cancer survivors that result from exercise training, including cardiopulmonary adaptations, endorphins distraction, mastery achievements, positive feedback, and social interaction Citation[44]. Further research that relates the observed changes in muscle strength to changes in quality of life is desirable.
The strengths of the present study include the supervised training program, the high adherence rates, and the validated measures of quality of life and physical performance. Furthermore, the intervention was longer than most exercise interventions in cancer patients, and data were collected at five time points to analyze training progress instead of only a pre- and post-treatment measurement. One limitation of the present study was the lack of a randomized controlled group. In the absence of a control group, it is necessary to differentiate the effects found from spontaneous recovery. An analysis of the correlation between the time delay from the last chemotherapy to the start of the training and the initial 1-RM and VO2 max values yielded no significant correlation. Consequently, the time variable has no effect on initial physical capacity. shows that the initial strength values were independent of the time between the last treatment and the start of the training. This analysis indicates that the observed effects on muscle strength could be attributed to the training intervention and not to the spontaneous recovery. Future randomized trials are necessary to confirm the results of the present study.
Finally, this study demonstrates a significant improvement in muscle strength, maximal oxygen consumption, and HRQOL after a high-intensity strength training program in cancer patients lasting 18 weeks. Muscle strength was related to physical functioning before and after training. Based on these findings, we recommend incorporating high-intensity strength training in cancer rehabilitation, with careful screening of patients and supervision during training.
Acknowledgements
Research foundation of the Máxima Medical Centre Hospital
References
- Courneya KS. Exercise in cancer survivors: An overview of research. Med Sci Sports Exerc 2003; 35: 1846–52
- Lucia A, Earnest C, Perez M. Cancer-related fatigue: Can exercise physiology assist oncologists?. Lancet Oncol 2003; 4: 616–25
- Dimeo F, Rumberger BG, Keul J. Aerobic exercise as therapy for cancer fatigue. Med Sci Sports Exerc 1998; 30: 475–8
- Smets EM, Garssen B, Schuster-Uitterhoeve AL, de Haes JC. Fatigue in cancer patients. Br J Cancer 1993; 68: 220–4
- de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs 2002; 25: 283–97
- Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer 2001; 92: 1689–93
- Berglund G, Bolund C, Fornander T, Rutqvist LE, Sjoden PO. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Eur J Cancer 1991; 27: 1075–81
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: Inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol 2000; 11: 971–5
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann Behav Med 1999; 21: 171–9
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol 2005; 23: 899–909
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients?. Eur J Cancer 2004; 40: 951–62
- McTiernan A. Physical activity after cancer: Physiologic outcomes. Cancer Invest 2004; 22: 68–81
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 2005; 23: 3830–42
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J Clin Oncol 2001; 19: 657–65
- van Weert E, Hoekstra-Weebers J, Otter R, Postema K, Sanderman R, van der SC. Cancer-related fatigue: Predictors and effects of rehabilitation. Oncologist 2006; 11: 184–96
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol 2002; 21: 1660–8
- Oldervoll LM, Kaasa S, Knobel H, Loge JH. Exercise reduces fatigue in chronic fatigued Hodgkin's disease survivors-results from a pilot study. Eur J Cancer 2003; 39: 57–63
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc 2002; 34: 1863–967
- Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effects of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum 1989; 16: 683–9
- Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003; 21: 1653–9
- Adamsen L, Midtgaard J, Rorth M, Borregaard N, Andersen C, Quist M, et al. Feasibility, physical capacity, and health benefits of a multidimensional exercise program for cancer patients undergoing chemotherapy. Support Care Cancer 2003; 11: 707–16
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol 2005; 23: 3577–87
- Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol 2005; 23: 2378–88
- Dimeo FC, Tilmann MH, Bertz H, Kanz L, Mertelsmann R, Keul J. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer 1997; 79: 1717–22
- Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Int J Biochem Cell Biol 2005; 37: 1084–104
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002; 2: 862–71
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 1993; 265: E210–E214
- Brill PA, Macera CA, Davis DR, Blair SN, Gordon N. Muscular strength and physical function. Med Sci Sports Exerc 2000; 32: 412–6
- Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. J Parenter Enteral Nutr 1986; 10: 558–63
- Schwartz AL. Cancer. ACSM's exercise management for persons with chronic diseases and disabilities, JL Durstine, GE Moore, et al. Human Kinetics, Champaign 2003; 166–173
- Kraemer WJ, Ratamess NA. Fundamentals of resistance training: Progression and exercise prescription. Med Sci Sports Exerc 2004; 36: 674–88
- Clinical exercise testing with reference to lung diseases: Indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing. European Respiratory Society. Eur Respir J 1997;10:2662–89.
- Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer 2001; 92: 1689–93
- Myers J. Applications of cardiopulmonary exercise testing in the management of cardiovascular and pulmonary disease. Int J Sports Med 2005; 26(Suppl 1)S49–S55
- Winningham ML. Strategies for managing cancer-related fatigue syndrome: A rehabilitation approach. Cancer 2001; 92: 988–97
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974; 32: 77–97
- Sale D, MacDougall D. Specificity in strength training: A review for the coach and athlete. Can J Appl Sport Sci 1981; 6: 87–92
- Mayhew JL, Prinster JL, Ware JS, Zimmer DL, Arabas JR, Bemben MG. Muscular endurance repetitions to predict bench press strength in men of different training levels. J Sports Med Phys Fitness 1995; 35: 108–13
- Meyer K, Samek L, Schwaibold M, Westbrook S, Hajric R, Beneke R, et al. Interval training in patients with severe chronic heart failure: Analysis and recommendations for exercise procedures. Med Sci Sports Exerc 1997; 29: 306–12
- Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: A randomized study. Acta Oncol 2005; 44: 449–57
- Johansson K. Is physiotherapy useful to the breast cancer patient?. Acta Oncol 2005; 44: 423–4
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2002; 34: 364–80
- Ott CD, Lindsey AM, Waltman NL, Gross GJ, Twiss JJ, Berg K, et al. Facilitative strategies, psychological factors, and strength/weight training behaviors at risk for osteoporosis. Orthop Nurs 2004; 23: 45–52
- Courneya KS. Exercise interventions during cancer treatment: Biopsychosocial outcomes. Exerc Sport Sci Rev 2001; 29: 60–4