Abstract
Eating dysfunction is a well-recognized consequence of orophagic tract cancers, but also occurs with other cancers. There is a relative absence of data assessing the impact of eating function on QoL in cancer populations other than those with disease of the oro-phagic tract. We assessed longitudinal changes in eating function and quality of life (QoL), and examined whether eating function predicted QoL over time in a sample of Chinese patients with breast, lung, liver, and nasopharyngeal cancers. Overall, 1 079 patients with breast, liver, lung, or nasopharyngeal carcinoma were assessed during their first outpatient visit (baseline) and at two follow-up interviews (FU1 and FU2). Three dimensions of eating function, including ability, appetite, and enjoyment, were assessed using three 11-point self-rated items. QoL was measured by the Chinese version of the Functional Assessment of Cancer Therapy-General Scale (FACT-G (Ch)). Linear mixed effects (LME) models evaluated mean differences on eating function and QoL scores across interviews and across cancer groups, and the effects of eating function on QoL. After adjustment for socio-demographic and medical variables, pain and depression, eating function significantly predicted patient overall (standardized βs ranged from 0.091 to 0.163, ps<0.05), physical (standardized βs ranged from 0.101 to 0.200, ps<0.05), and functional (standardized βs ranged from 0.120 to 0.162, ps<0.05) aspects of QoL scores over time. Eating dysfunction significantly impacts QoL in cancer populations other than those with orophagic disease. Change of eating function appears to be a common problem in cancer patients regardless of cancer site.
Eating dysfunction commonly follows head and neck (H&N) and esophageal cancer and related treatments Citation[1–4], compromising short and medium term quality of life (QoL) Citation[5], Citation[6]. In two major QoL instruments, the European Organization of Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) Citation[7] and the Functional Assessment of Chronic Illness Therapy (FACIT) Citation[8], eating was conceptualized as a multidimensional construct incorporating appetite, swallowing, eating ability and enjoyment (Appendix 1). The generic FACIT core items do not include eating, but 12 of the 17 site-specific FACIT subscales assess appetite. Additionally, the site-specific subscales for esophageal, gastric, and H&N cancers also evaluate swallowing, eating ability, and enjoyment, with more than half of the items in the esophageal subscale measuring eating function. The generic EORTC QLQ, C30, includes one item assessing appetite. Of the seven validated site-specific modules, the H&N, oesophageal, and stomach modules contain at least eight items assessing eating function.
Conventionally, eating disruption among esophageal, gastric, stomach, and H&N cancer is grounded in the direct impairment caused as these tumors derange eating function. However, other influences on eating may impair QoL in patients with cancer. Eating dysfunction is prevalent among lung cancer patients Citation[9], Citation[10], while change of appetite predicted higher severity of depression among patients with cancers that directly (e.g., stomach, H&N, esophagus) and indirectly (e.g., lung, breast, colon, and pancreas) influenced eating ability Citation[11]. Eating-related somatic symptoms emerged as an independent factor underlying depressive symptoms in ambulatory cancer patients Citation[12]. Among Chinese nasopharyngeal cancer patients, eating ability significantly predicted QoL Citation[13]. Appetite loss in a mixed sample of cancer inpatients was second only to fatigue in prevalence, with 32% of patients reporting loss of interest in food. The authors concluded that lack of food intake alone can impair QoL Citation[14]. After role and social function loss, nausea and vomiting, and fatigue, appetite loss was the side-effect hospitalized cancer patients wished to most avoid Citation[15]. Eating dysfunction is a common side-effect of cancer treatment and a physiological response to cancer, particularly in more advanced disease, contributing to cachexia. Uncertainty arising from a relative absence of data remains regarding any significant psychological effect of eating dysfunction in a variety of non-orophagic cancers.
We describe the impact of eating function on subsequent QoL in a prospective study with 6-month follow-up. We prospectively assessed the longitudinal relationships between eating function and QoL among Chinese patients with breast, liver, lung, or nasopharyngeal carcinoma. We examined changes in eating function and QoL and tested the hypothesis that eating function predicted QoL over time.
Methods
Study sample
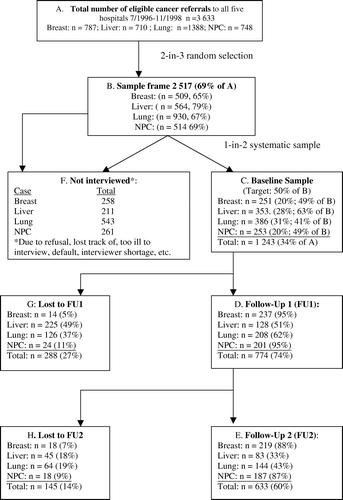
Following IRB approval, patients attending clinical oncology outpatient clinics at the five largest regional hospitals in Hong Kong were eligible for study participation if they met the following criteria: (1) histologically or cytologically documented primary breast, liver, lung (small cell or non-small cell), or nasopharyngeal carcinoma, (2) between 19 to 85 years of age, (3) native Cantonese speakers, (4) having no Axis I mental disorder, and (5) having no communication problems or physical conditions that would prevent the completion of the interview.
Procedures
As part of a larger QoL study Citation[13] all new referrals to the five radiation-oncology clinics involved in the study with confirmed diagnosis of breast, liver, lung, or nasopharyngeal cancer during the period of data collection (between July 1996–September 1997 and November 1998) comprised the sample frame. All patients had completed primary surgical treatment on referral. During 1997, of 11 086 new cancers of all categories recorded by the Hong Kong Cancer Registry (HKCR), there were a total of 8 234 new cases of these four cancer groups of which 45.8% comprised lung, 20.6% liver, 19.5% breast and 13.9% nasopharyngeal cancer cases. The five clinics had total of 3 663/8 234 of these new cases referred during the recruitment period, comprising 1 388 lung (36.8% of all new lung cancers recorded by the HKCR in 1997), 710 liver (41.7% of all new HKCR liver cases), 787 breast (49% of new HKCR breast cases) and 748 nasopharyngeal cases (65.3% of all new nasopharyngeal cases). The sample frame was a 2 in 3 random subset (69%) of all eligible patients, from whom 1 in 2 (34%) were targeted for recruitment within a defined time period to minimize effects from evolution in clinical management. Liver and lung cancer patients were oversampled to allow for higher attrition during follow-up. After participants gave informed consent, trained interviewers performed face-to-face interviews in private using Cantonese with all participants at baseline recruitment using a standardized structured questionnaire immediately following each patient's clinical consultation. Inter-rater reliability was evaluated by simultaneous documentation of a random sub-set of interviews periodically over the course of the data collection period and was found to be above 0.9, reflecting minimal inter-rater drift Citation[13]. Follow-up interviews were performed either face-to-face in a private room in a clinic setting while respondents were waiting scheduled consultations or were done via telephone interview.
Measures
Socio-demographic and clinical variables
Data on patients’ socio-demographic information were collected during baseline interviews. Clinical data were extracted from patients’ medical record using a standardized form by a medically qualified researcher following a standardized protocol. Because of recording problems within and logistical limitations accessing medical records, stage at diagnosis and recurrence and treatment after diagnosis were the measures used to accommodate variations in clinical status (see Data Analysis section).
Quality of Life (QoL)
QoL was assessed with the Chinese version of the Functional Assessment of Cancer Treatment (FACT-G) version-3 scale (FACT-G (Ch)), which is consisted of 27 items scoring on a 5-point scale (0 = not at all, 4 = very much). Scores were added for a total QoL assessment score (Tot). The FACT-G (Ch) has four subscales, including physical well-being (Physical), emotional well-being (Emotional), functional well-being (Functional), and social/family well-being (Social/Family). The FACT-G (Ch) is regarded as reliable and valid for studies of adult Hong Kong Chinese cancer patients Citation[13], Citation[16–19].
Eating function
Three dimensions of eating function were evaluated using the statement “My eating ability is …” (Eating Ability), “My eating appetite is …” (Eating Appetite), and “I enjoy eating” (Eating Enjoyment) respectively. The items were rated on a 11-point scale, with the “0” end headed “very bad”/“do not enjoy at all” and the “10” end “very good”/“enjoy very much”.
Pain
Pain can impair QoL Citation[18], so three pain items were included as covariates in statistical modeling Citation[20–22]. Using a white plastic rule graduated in 11 points labeled 0–10, along which a red pointer slides revealing a red bar respondents were asked to indicate a point corresponding to “Your pain level right now” (Current Pain), “Your pain on average in the last month” (Average Pain), and “Your pain at its worst in the last month” (Worst Pain). Respondents were told that a rating of “0” denoted “no pain at all” while a “10” was “pain so severe as to prohibit all activity; the worst pain you can imagine”. Scores were then transferred by the interviewer to an eleven-point (0–10), 10 cm visual analogue (VA) scale labeled “0” and “10” at opposite ends. This was later coded to an integer from 1–10.
Depression
“Depression” was assessed using a single item likert-type statement “I am depressed”, which was rated on a 5-point scale (0 = very much, 4 = not at all). This single item measure was used to minimize assessment burden on the patients with more deteriorated condition, and utilized in statistical modeling as a covariate to adjust for depression Citation[19], Citation[20].
Data analysis
Standard descriptive analyses (mean and standard deviation [SD]) assessed sample characteristics. Socio-demographic and medical differences between cancer groups were determined using chi-square tests for proportions or one-way analysis of variance (ANOVA) for continuous measures. Disease stage was dichotomized as “Early” or “Advanced” to accommodate the different staging systems in use, with “Early” corresponding to Stages 1 and 2 (Liver), and Stages 0–IIB and “early” for non-small cell and small-cell lung cancers respectively, stage 0–II for Breast and Nasopharyngeal. “Advanced” included all other stages. Mean differences on QoL, eating function, pain, and depression measures across time and between cancer groups were analyzed using linear mixed effects (LME) models, with random subject effects estimated for the intercept, slope for time (interval between interviews in months), and time squared (time2). The quadratic effect of time was included to account for nonlinear change over time. It was anticipated that change would likely not be linear; thus time2 was included to avoid systematic overestimation or underestimation of data points. To determine whether eating associated with QoL scores, five LME models were fitted to each outcome, including Total (Model 1), Physical (Model 2), Social/Family (Model 3), Functional (Model 4), and Emotional (Model 5) respectively. All 5 LME models were fully adjusted for relevant demographic and clinical variables, pain and depression, factors known to potentially influence eating behaviour or QoL in cancer patients Citation[19]. The primary variables of interest in the analyses were time, time2, cancer site, and the three eating function variables. Nasopharyngeal cancer treatment directly impacts oral physiology and hence eating function and so Nasopharyngeal was the referent in LME models comparing cancer site. All LME models used standardized scores of QoL, eating function, pain, and depression. The LME analyses were performed on all data collected on the three time points, thus using all information available. SPSS version 11.0 implemented all analyses.
Results
Sample characteristics
Of 3 633 eligible patients, 2 517 (69%) formed the sample frame and one in two sampling thereof produced 1 243 patients (50%) (), of whom 1 079 provided complete data and were included in the present analysis. summarizes their baseline socio-demographics and clinical characteristics. Frequencies of characteristics at FU1 and FU2 were also examined. Proportions stayed relatively constant from baseline through FU1 and FU2. At baseline, the mean age for the entire sample was 55.94 (SD = 13.53), with Lung patients being significantly older (F = 126.5, p<0.001). All Breast patients were women while males predominated in the remaining three groups (≥73%, F = 448.5, p < 0.001). The proportions of Lung patients completing primary/secondary education (69%) (F = 42.0, p < 0.001) and married/cohabiting (78%) were significantly lower than that of other groups (F = 27.8, p < 0.005). More of the Liver (87%) and Lung (73%) groups had more advanced disease than did Breast (16%) and Nasopharyngeal (11%) at diagnosis (F = 442.8, p < 0.001). Among the Lung patients, 88% had non-small cell cancer. Most patients (≥77%) had no recurrence after baseline, though Lung patients (24%) were more likely to develop a recurrence after baseline (F=45.034, p < 0.001). Between baseline and FU1, patients in the Breast (90%) and Nasopharyngeal (96%) group were more likely to have further treatment (F = 119.2, p<0.001) while between FU1 and FU2 Breast patients (76%) were more likely to have received treatment compared with the other groups (≤22%) (F = 198.2, p < 0.001) An average of 2.99 months (SD=1.02) and 3.01 months (SD=1.19) had elapsed between baseline and FU1, and between FU1 and FU2 respectively for the entire sample. Liver patients moreover had a significantly shorter interval between the three assessments (ps < 0.001). Proportion of patients completed FU1 assessment using telephone interview ranged from 27.3% (Nasopharyngeal) to 60.2% (Liver), and the rates were generally increased at FU2 for all of the sample, except Liver. Student's t-tests found no significant differences between face-to-face interview and telephone interview on QoL, eating, and pain scores at either follow-up assessment (p > 0.05).
Table I. Socio-demographic and clinical characteristics of the study sample at baseline (n = 1 079).
Mean differences on QoL, eating, pain, and depression across times and across groups
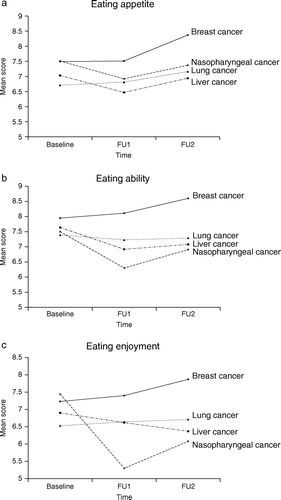
summarizes the mean comparisons of QoL, eating, pain, and depression scores across time and groups. LME analyses indicated that Breast patients scored significantly higher than Nasopharyngeal patients on Functional (β=0.311, p < 0.001), eating ability (β=0.753, p < 0.001), eating appetite (β=0.348, p < 0.001), eating enjoyment (β=0.753, p < 0.001), but lower on depression (β= − 0.152, p < 0.05) (also see ). Both Liver and Lung patients scored significantly lower than Nasopharyngeal patients on Total (Liver: β= − 0.532, p < 0.001; Lung: β= − 0.649, p < 0.001), Physical (Liver: β= − 0.456, p < 0.001; Lung: β= − 0.526, p < 0.001), Functional (Liver: β= − 0.473, p < 0.001; Lung: β= − 0.677, p < 0.001), Emotional (Liver: β= − 0.358, p < 0.001; Lung: β= − 0.296, p < 0.001), eating appetite (Liver: β= − 0.357, p < 0.001; Lung: β= − 0.465, p < 0.001), and depression (Liver: β= − 0.403, p<0.001; Lung: β= − 0.351, p < 0.001). Lung patients also reported significantly higher levels of pain (β=0.311, p < 0.001) and average pain (β=0.216, p < 0.05) than did Nasopharyngeal patients. LME indicated non-linear changes over time on Social/Family (β=0.016, p < 0.05) and average pain (β=0.013, p < 0.05). Mean scores on worst pain differed significantly over time (β=0.014, p < 0.05), for Breast (β= − 0.187, p < 0.05) and Lung (β=0.262, p < 0.001) compared to the Nasopharyngeal sample. Significant linear change was observed for reported eating ability (β=0.117, p < 0.05).
Table II. Linear mixed effects analysis: Mean comparisons of QoL, eating, pain, and depression scores across times and across cancer groups.
Linear mixed effects models for QoL scores
reports the significant predictors of five LME models fit to Total (Model 1), Physical (Model 2), Social/Family (Model 3), Functional (Model 4), and Emotional (Model 5). The absence of interaction effects indicated that QoL did not change significantly over time, whether in a linear or curvilinear fashion, for any group. Cancer site significantly predicted QoL scores in Models 1, 2, and 4 (all ps < 0.05), with Lung consistently predicted poorer scores (Total (β= − 0.324, p < 0.001), Physical (β= − 0.198, p < 0.05), and Functional (β= − 0.379, p < 0.001)) than Nasopharyngeal. Liver and Breast predicted lower scores than Nasopharyngeal on Total (Model 1, β= − 0.300, p < 0.05) and Physical (Model 2, β= − 0.221, p < 0.05) respectively. Depression was associated significantly with QoL in all models, with standardized betas ranging from 0.15 (Model 3, p < 0.001) to 0.644 (Model 5, p < 0.001). Eating ability, eating appetite, and eating enjoyment predicted Total, Physical, and Functional (ps < 0.05), but not Emotional. Eating appetite also predicted Emotional (β=0.117, p<0.005). Worst pain was associated with subsequent Total (β= − 0.174, p < 0.001), Physical (β= − 0.240, p<0.001), and Social/Family (β= − 0.118, p < 0.05). Other pain variables were unassociated with later QoL.
Table III. Linear mixed effects models for FACT-G (Ch) scores.
Discussion
We know of no prior longitudinal model that evaluated eating function over time, or impact on QoL among Chinese patients with breast, liver, lung, and nasopharyngeal cancer. The four cancer groups differed significantly on QoL dimensions and eating function, with the Breast group displaying significantly better QoL and eating function. Eating predicted Total, Physical, and Functional aspects of QoL scores. In particular, eating impacted QoL differently across the four cancer groups, with the Lung group consistently displaying greater negative effects of eating function on Total, Physical, and Functional aspects of QoL relative to the Nasopharyngeal group. Consistent with previous research, depression emerged as a significant predictor of all QoL scores Citation[23], Citation[24].
In QoL, eating dysfunction has largely been specific to oro-phagic tract cancers. Our results suggest that in this sample of patients with non-orophagic cancers, eating ability, eating appetite, and eating enjoyment were positively associated with the overall, physical and functional aspect of QoL, remaining so even after adjusting for major clinical variables, pain, and depression. These results are in line with prior reports Citation[11], Citation[12], indicating that changes in eating may not be disease specific. It is well known that systemic effects of malignancy commonly impact eating in cancer patients regardless of cancer site, but this is generally not considered in QoL instruments. The significant impact of eating function on the Physical and Functional subscales of FACT-G (Ch) demonstrates its contribution to physical and functional well-being. Eating therefore appears to be a useful core domain in evaluating QoL among a range of cancers and should be comprehensively assessed in QoL instruments for non-orophagic cancers as well. While eating function changed little over time, the significant decrements of eating function among Nasopharyngeal patients as compared to other cancer samples lends further support to the clinical validity of the three eating measures.
Since eating dysfunction, especially loss of appetite, is a common somatic symptom of depression, the association between eating function and QoL reported in the present study could be confounded by depression. Consequently, post hoc analyses examined the correlations between the three eating variables and depression at the three assessment points. Eating-depression correlations suggest a weak to moderate relationship between depression and eating, (r range: 0.190–0.344, all p < 0.001), implying depression only partly accounted for variation in eating function in the present data. Other factors remain unaccounted for.
Variability in QoL and eating function over time were insignificant. These results may be partly explained by the small proportion of the current sample (ranging between 6% in the Liver and 23% in the Lung sample) having had recurrence during the study interval. Using different QoL measures and collecting other corroborative data such as functional status, future investigation should verify to what extent the lack of variability in QoL and eating scores over time is attributed to measurement sensitivity or the general good prognosis of the sample.
Pain, widely recognized as an important influence on QoL, did selectively impact QoL scores in the current sample. Though lower worst pain was associated with better overall, physical, and social/family functioning, current and average pain did not predict any QoL scores. In this sample, eating function appears to be a more useful domain than pain for evaluating QoL. Future studies might focus on worst pain as the most influential pain variable affecting QoL. These findings may be understood in the light of cultural influence on how people perceive the constituents of QoL and health in general Citation[25], and the fact that food and eating are dominant concerns for Chinese people Citation[26] and in many other cultures too. Patients with different cancer diagnoses, stages of disease and treatment exhibit different QoL domains/symptoms that they most wished to avoid worsening Citation[15]. Research is needed to determine to what extent cultural beliefs regarding food and eating contribute to eating problems that in turn affect QoL. This may also reflect problems with generic measures of QoL, such as the FACT-G (Ch).
Despite the significant findings from this study, the relationship between eating and QoL in non-orally-related cancer types should be considered tentative. Many unanswered questions remain about the nature of eating's contribution to the construct of QoL, particularly within non-orally related cancer populations. Clarification of the extent to which eating is a unique or a generic domain in QoL measures is needed. Future investigations should also explore how various dimensions of eating (e.g., eating ability, appetite, enjoyment, and swallowing) co-vary across the illness trajectory and among various treatments, and whether they should be considered as separate or inter-linked. An examination of these issues will help to clarify the utility of eating as a QoL domain in different cancer populations. Finally, while the present data suggest eating is not adequately portrayed by FACT-G (Ch) in assessing QoL among Breast, Liver, Lung, and Nasopharyngeal patients, a comparison using other QoL instruments, such as EORTC QLQ, and other oncology populations would be informative. Use of single items to assess depression may limit the conclusiveness of the findings obtained in this study. However, previous research showed that single-item measures of depression (“Do you often feel sad or depressed?”) accurately classified more than 80% of elderly patients Citation[27] or patients with stroke Citation[28], and both sets of findings were confirmed using standardized depression scales. Future attempts to replicate or validate the current findings employing standardized scales to tap depression which is assumed to be multidimensional, would be more robust.
Acknowledgements
This project was supported by grants from the Hong Kong Government Health Services Research Committee (HSRC #821005) and a donation from Mr. C. S. Suen. The following people contributed to the study in different ways and at different times and their help is acknowledged: LM Ho PhD, PHK Choi FRCR, DTK Choy FRCR, WYC Foo, WH Lau FRCR, AWM Lee, SF Leung, SKO FRCR, Dr. JST Sham FRCR, VKC Tse, FRCR, KH Wong, Professor CLW Chan for suggestions regarding the questionnaire, and Dr. CLM Yu whose efforts in coordinating the project are deeply appreciated. Finally, we thank all patients and their families.
References
- Beeken L, Calman F. A return to “normal eating” after curative treatment for oral cancer. What are the long-term prospects?. Eur J Cancer 1994; 30B: 387–92
- Pauloski BR, Logemann JA, Rademaker AW, McConnel FM, Stein D, Beery Q, et al. Speech and swallowing function after oral and oropharyngeal resections: One year follow–up. Head Neck 1994; 16: 313–22
- Lazarus CL, Logemann JA, Pauloski BR, Colangelo LA, Kahrilas PF, Mittal BB, et al. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope 1996; 106: 1157–66
- Rothwell JF, Feehan E, Reid I, Walsh TN, Hennessy TP. Delay in treatment for oesophageal cancer. Br J Surg 1997; 84: 690–3
- Karnell LH, Funk GF, Hoffman HT. Assessing head and neck cancer patient outcome domains. Head Neck 2000; 22: 6–11
- Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and treatment of Cancer Quality of Life Questionniare-H&N35. J Clin Oncol 1989; 17: 1008–19
- EORTC Quality of Life. Retrieved June 2, 2006, from http://www.eortc.be/home/qol/default.htm.
- FACIT: Functional Assessment of Chronic Illness Therapy Retrieved June 2, 2006, from http://www.facit.org/about/welcome.aspx.
- Degner L, Sloan J. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage 1995; 10: 423–31
- Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress and change over time in adults receiving treatment for lung cancer. Psycho-Oncology 2003; 12: 694–708
- Akechi T, Nakano T, Akizuki N, Okamura M, Sakuma K, Nakanishi T, et al. Somatic symptoms for diagnosing major depression in cancer patients. Psychosomatics 2003; 44: 244–8
- Passik SD, Lundberg JC, Rosenfeld B, Kirsh KL, Donaghy K, Theobald D, et al. Factor analysis of the Zung Self-Rating Depression Scale in a large ambulatory oncology sample. Psychosomatics 2000; 41: 121–7
- Yu CLM, Fielding R, Chan CLW. The mediating role of optimism on post-radiation quality of life in nasopharyngeal carcinoma. Qual Life Res 2003; 211: 41–51
- Trabal J, Leyas P, Forga MT, Hervas S. Quality of life, dietary intake and nutritional status assessment in hospital admitted cancer patients. Nutricion Hospitalaria 2006; 21: 505–10
- Osoba D, Hsu MA, Copley-Merriman C, Coombs J, Johnson FR, Hauber B, et al. Stated preferences of patients wit cancer for health-related quality of life domains during treatment. QoL Res 2006; 15: 273–83
- Yu CL, Fielding R, Chan CLW, Tse VK, Choi PH, Lau WH, et al. Measuring quality of life of Chinese cancer patients: A validation of the Chinese version of the Functional Assessment of Cancer Therapy-General (FACT-G) scale. Cancer 2000; 88: 1715–27
- Yu CL, Fielding R, Chan CLW, Sham JST. Chinese nasopharyngeal cancer patients treated with radiotherapy: The association between information satisfaction and quality of life. Cancer 2001; 92: 2126–35
- Wong WS, Fielding R. Quality of life and pain in Chinese lung cancer patients: Is optimism a moderator or mediator?. QoL Res 2007; 16: 53–63
- Wong WS. Fielding R. Change in quality of life in Chinese women with breast cancer: Changes in psychological distress as a predictor. Support Care Cancer (in press).
- Prucha E. Health reference series: Cancer sourcebook3rd ed. Omnigraphics, Detroit 2000
- Cook A. Health reference series volume 12: The new cancer source book. Omnigraphics, Detroit 1996
- Anie KA, Steptoe A, Bevan DH. Sickle cell disease: Pain, coping and quality of life in a study of adults in the UK. Br J Health Psychol 2002; 7: 331–44
- Weitzner MA, Meyer CA, Stuebing KK, Saleeba AK. Relationship between quality of life and mood in long-term survivors of breast cancer treated with mastectomy. Support Care Cancer 1997; 5: 241–8
- Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio SW, Frierson GM, Jim HS, Carpenter KM, et al. Traumatic stress, perceived global stress, and life events: Prospectively predicting quality of life in breast cancer patients. Health Psychol 2005; 24: 288–96
- Marshall PA. Cultural influences on perceived quality of life. Semin Oncol Nurs 1990; 6: 278–84
- Gabrenya WK, Hwang KK. Chinese social interaction: Harmony and hierarchy on the good earth. In: Bond MH The handbook of Chinese psychology. Hong Kong: Oxford University Press; p 309–321.
- Mahoney J, Drinka TJK, Abler R, Gunter-Hunt G, et al. Screening for depression: Single question versus GDS. J Am Geriatr Soc 1994; 42: 1006–8
- Watkins C, Daniels L, Jack C, Dickinson H, van Den Broek M. Accuracy of a single question in screening for depression in a cohort of patients after stroke: Comparative study. BMJ 2001; 323: 1159
Appendix 1. Number of Items Assessing Eating Function in FACIT and EORTC QLQ.