Abstract
Objective. To compare 14-gauge SCNB (stereotactic core needle biopsy) with surgery and to investigate tissue-heterogeneity of estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth-factor receptor (HER-2) for non-palpable breast cancers. To determine the number of cores needed for assessment of these factors. Materials and methods. Cores of 41 invasive cancers were collected in three containers: the 1st into A, the 2nd and 3rd into B and subsequent cores into C. ER, PR and HER-2 were scored by immunohistochemistry and if 2+ or 3+, by chromogenic-in-situ-hybridisation (CISH) for containers and for surgical specimen. Results. Between SCNB and surgical specimen concordance was 83% (κ=0.39) for ER, 88% (κ=0.69) for PR and HER-2 and 93% (κ=0.63) for HER-2 after CISH. For the most discordant cases, status was positive in cores but negative in surgery: 5/7 for ER (p=0.459), 5/5 for PR (p=0.063), and 4/5 for HER-2 (p=0.375), after CISH 3/3 (p=0.250), but the difference was not statistically significant. Concordances between containers of cores was 100% (κ=1), 85% (κ=0.66) and 85% (κ=0.66), respectively. With more than three cores, sensitivities of 95%, 100% and 100% were reached. Conclusions. SCNB is at least as sensitive as surgery in assessment of ER, PR and HER-2. Three cores are needed for reliable assessment of HER-2 after adding CISH and more than three cores for PR, possibly due to tissue heterogeneity. For ER sensitivity remained lower, 95%, even in multiple cores, therefore ER-negative cases should be further investigated from surgical specimens.
The incidence of breast cancer is increasing worldwide, which increases the popularity of mass screening programmes and leads to the discovery of clinically occult breast lesions. Stereotactic core-needle breast biopsy described by Parker et al. Citation[1] is widely used for the evaluation of non-palpable breast lesions. In addition to high sensitivity and specificity in confirming malignant tissue in suspicious breast lesions Citation[2], core samples also allow preoperative determination of histological prognostic factors such as grade, type, and invasion of neoplasm, as well as ER, PR and HER-2 status by immunohistochemistry (IHC) Citation[3–5].
Hormone receptor and HER-2 assessments are essential for treatment decisions in breast cancer. Antiestrogen treatment plays a central role of both adjuvant therapy and therapy for advanced breast cancer in patients with ER and/or PR positive tumours Citation[6], Citation[7]. HER-2 overexpression has been shown to be associated with response to trastuzumab (a humanised recombinant monoclonal IgG1 antibody against HER-2, Herceptin, Roche) anticancer therapy, as well as with aggressive disease and poor prognosis Citation[8], Citation[9]. Until now trastuzumab has been indicated for use in advanced disease, but the recently published large multi-centre clinical trials with promising preliminary results have increased the propensity of its use as adjuvant treatment for early breast cancer. For the correct treatment decision in this setting it is utterly important that a reliable HER-2 assessment can be done Citation[10–12].
A few studies have been performed comparing prognostic information on core samples with subsequent surgical specimen Citation[5], Citation[13–16]. The applicability of core samples has been shown. However, the question of tumour heterogeneity is the main drawback in evaluating core samples for preoperative use. It remains uncertain if the cores obtained are representative for the whole tumour Citation[3], Citation[5] since the distribution of antigens could be heterogeneous within the tumour. Therefore, the surgical specimen has traditionally been the gold standard in the assessment of prognostic factors of breast cancer.
The aim of our prospective study was to compare core samples obtained by stereotactic device and surgical specimens to determine the most accurate method for the assessment of ER, PR and HER-2 and thus to minimise the unnecessary withholding of at best, a life saving treatment for the patient. In addition, we wanted to investigate in a clinical setting the heterogeneity of non-palpable breast cancers for ER, PR and HER-2 from different containers of cores and also to determine how many 14-gauge add-on stereotactic core samples are needed for a reliable assessment of these prognostic factors.
Materials and methods
Patients from four screening centres, two district hospitals and tertiary care centres are referred to our hospital (catchment area 251 000 people) for stereotactic core breast biopsy. Between June 1998 and January 2001, 661 patients with mammographically detected suspicious breast lesions were referred. Ultrasound guided core needle biopsy was performed for 449 patients. Lesions that could not be detected by ultrasound (small, nonpalpable mass lesions, architectural distortions and clusters of microcalcifications, altogether 212 patients) were scheduled for core biopsy using an add-on stereotactic biopsy device. The study was approved by the ethical committee of the hospital. Informed consent was obtained.
The biopsy material was collected as follows: the first sample (the central needle pass) was collected into container A, the second and the third passes (2 mm from the centre) were collected into container B, and all additional samples were collected into container C. The mean number of biopsies placed in container C was 4 (range, 1–11). Histopathology and reporting were performed for each container separately. After core-needle biopsy, all the women either underwent surgical excision, or if the diagnosis from stereotactic core biopsy was benign, were followed up with mammography. The time interval between core biopsy and surgery was 14 days (range, 3–24 days). For the comparison of both different containers of cores and core samples with surgical specimen, only patients with invasive carcinoma both in core biopsies in at least two containers and in surgical specimen were included in the study.
Of the 212 consecutive patients with 220 non-palpable, mammographically detected breast lesions eligible for this study, 15 patients were excluded. In seven of the excluded patients the location of the lesion was high, near the axillary fossa or so close to the thoracic wall and pectoral muscle that it could not be reached by the stereotactic equipment. Such lesions were excised surgically. In two (1%) patients the biopsy had to be terminated after the first pass because of a vaso-vagal reaction. In five patients the samples had all been placed in one container instead of three containers, so that separate analysis could not be performed. In addition, one patient died from unrelated causes before any follow-up examination. Thus 197 patients (mean age 56 years, range 32–88 years) with 205 lesions were included in the study. From 54 (27%) patients with invasive carcinoma in the surgical specimens, 13 patients with invasive carcinoma detected only in 0 to 1 containers were excluded from the study: 11 patients had either ductal carcinoma in situ (DCIS) (n = 7), atypical ductal hyperplasia (ADH) (n = 3) or benign findings (n = 1) in the three containers and two patients had invasive carcinoma only in one container. Altogether 41 patients with invasive breast carcinoma in at least two containers and in surgical sample were included in this study (). In three cases the first container (A) did not contain invasive carcinoma, in three cases the second container (B) did not contain invasive carcinoma and in one case no invasive carcinoma was detected from the third container (C). IHC-assessments for ER, PR and HER-2 were performed separately for each container and for the surgical sample.
Table I. Patient and lesion characteristics.
Stereotactic core needle biopsy (SCNB)
All biopsies were performed using a regular mammography machine (Sophie, Planmed, Helsinki, Finland) and an add-on stereotactic biopsy device (Cytoguide, Planmed, Helsinki, Finland) with the patient in an upright seated position. An automated biopsy gun (C.A. Bard, Covington, GA) with a 22-mm throw and a 14-gauge needle was used. The biopsy procedures were performed by one of five radiologists (M.S., M.B. and three others), all with 4–6 years of experience in taking breast biopsies. After localising the lesion the first needle pass was targeted to the centre of the lesion. Routine pre-fire stereotactic views were obtained to confirm the position of the needle, which was modified if needed to ensure the central position. The following biopsies were obtained in a clock-wise direction 2 mm from the centre (for 3–4 biopsies) and the rest of the passes more distally from the centre. For microcalcifications there was more variability in needle placement. The intent was to target the most suspicious area. The first pass was obtained either by targeting the centre of the lesion or by selecting a particularly distinctive calcification that could be reliably discerned on the two stereotactic images. Subsequent passes were planned according to the geography of the calcifications. The overall biopsy procedure was accomplished as previously described Citation[17].
Patients received no radiation therapy or medical treatment for breast cancer between stereotactic core biopsy and surgery. Clinical data was collected from patient records.
Histology of the samples
Tumours were staged according to TNM classification and histological grading was performed according to the Nottingham modification of the Bloom and Richardson method.
Handling of the histological specimen was standardised. Core samples were immediately put to 10% neutral formalin and sent to the Department of Pathology, where they were kept in fixative 2 to 4 hours before routine processing overnight. The fresh surgical specimen was x-rayed to ensure that the lesion was in the sample with margins (time delay half an hour). Then the specimen was measured, painted and sliced within the next half an hour. After overnight formalin fixation the specimen were cut, processed and embedded in paraffin on the following day.
All the hematoxylin eosin slides, IHC and CISH stainings were reviewed by the same consultant pathologist (VK) with 7 years experience in breast pathology. Double reading (VK, JR) was performed for HER-2 staining. In cases of discrepancy a consensus reading was done. The IHC and CISH methods are described in detail in the appendix.
For ER and PR, the proportion of nuclear staining was quantified from 0 to 100%. Staining < 10% was further designated as negative and ≥10% as positive. For HER-2, the Clinical Trial Assay (CTA) system was used to grade the degree of membrane staining. No staining or membrane staining observed in less than 10% of tumor cells was given a score of 0. A faint /barely perceptible membrane staining detected in more than 10% of the tumor cells was scored as 1+ and a weak to moderate membrane staining in >10% was graded as 2+. Strong complete membrane staining in >10% of the tumor cells was graded as 3+ which was a threshold for HER-2 positivity. All 2+ and 3+ results were further investigated by CISH to detect gene amplification. The gene copy number was defined as unaltered when there were one to five signals per nucleus. Amplification was defined as six or more signals in the nucleus, or as at least a small gene copy cluster in over half of the cancer cells.
Statistics
For ER, PR and HER-2, agreement between different containers of cores and between core samples and surgical specimen were determined using ER-, PR- and HER-2 assessments dichotomised as positive or as negative. Containers were dichotomized as negative if all cores in the container were negative and positive if at least one of the cores was positive for ER, PR and HER-2. Container combinations were designated accordingly; negative if all containers were negative and positive if at least one of the containers was positive. Agreement was tested by the kappa statistics.
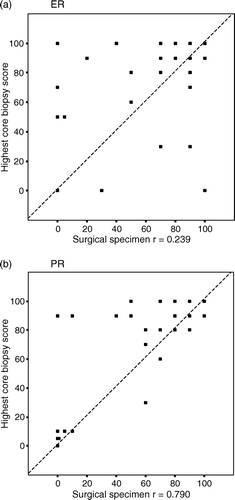
Continuous variables were tested for normal distribution with the Kolmogorov-Smirnov 1-sample test. Statistical differences in the proportions of positive and negative cases between the dichotomised ER, PR and HER-2 scores in core samples and surgical specimen were evaluated using McNemar's non-parametric paired proportions test. The highest ER-expression and PR-expression values among the three containers of cores were selected and further paired with the ER and PR-expressions of the corresponding excised tumour by using the Wilcoxon Signed Ranks Test.
Sensitivities of the individual containers (A, B and C) and container combinations (A + B, A + B+C) for the assessment of ER, PR and HER-2 were calculated. In the calculations of sensitivity, the “optimal reference” was used, which was considered positive if at least one of the containers or surgical specimen was positive and negative if all three samples and surgery were negative.
The false negative rate was determined for ER, PR and HER-2 assessments separately for core biopsies and for surgical samples by dividing the number of cases that were negative by the method (core biopsy or surgical sample) and positive by the other (reference) method by all positive cases of the reference method.
For ER and PR, Spearman correlation coefficients were calculated for the proportions of ICH positive cells relative to all malignant cells both for separate core samples and for core samples and surgical specimen.
All data were analysed using the statistical package SPSS for Windows, Version 11.5. (SPSS, Inc., Chicago, IL). P-value <0.05 (two-sided tests) was considered statistically significant.
Results
Comparison of core samples with surgical specimen
The agreement between core samples and surgical specimens was high: 83% for ER, 88% for PR, 88% for HER-2 (IHC) and 93% after adding CISH (). Discordant cases tended to be positive for core samples, but negative for surgery: 5 of 5 discordant cases were positive in core biopsies for PR, 5 of 7 for ER, 4 of 5 for HER-2 after IHC and 3 of 3 for HER-2 after IHC and CISH, respectively. However, these differences did not reach statistical significance ().
Table II. Agreement, number of discordant cases and percentages of positive stainings in core samples compared to surgical specimen.
Cases showing 2+ or 3+ HER-2 overexpression by immunohistochemistry (9 (22%) surgical specimens and 19 (46%) core biopsy samples) were further investigated by CISH. HER-2 gene amplification was detected in six cases according to core biopsies but only in three cases for surgical specimens.
When comparing the prognostic assessment based on the surgical specimens to that based on the core samples, five false negative assessments were made for ER (14%) and PR (15%) and three false negative assessments for CISH-positive HER-2 (50%). When comparing prognostic assessments made from core samples with assessments made from surgical specimens, two false negative assessments were encountered for ER (6%), and none for PR and HER-2. Proportions on immunohistochemically PR-positive and ER-positive cells from all malignant cells in core samples (highest score) versus surgical specimens cells were significantly higher in core biopsies than in surgical specimens (for ER 74% vs. 63%; p = 0.031, for PR 62% vs. 50%; p = 0.001) ().
Comparison of containers of cores
When dichotomized as positive or negative, ER assessments for all three containers were concordant in 41/41 (100%) of the cases (κ = 1.0) and PR and HER-2 assessments in 35/41 85% of the cases (κ = 0.66).
The proportions of ER and PR positive cells relative to all malignant cells in each container are shown in . The containers of cores show a high correlation for PR. In discordant cases the proportions are near the cut-off value. For ER more heterogeneity was detected between different containers. Sensitivities for the detection of ER, PR and HER-2 were individually calculated for the three containers and container combinations (). The central core (container A) did not differ markedly in sensitivity from the more peripheral cores (containers B and C) in the assessment of ER, PR and HER-2. With three cores sensitivity was 95% for ER, 92% for PR, 95% for HER-2 (IHC) and 100% after adding CISH. With more than three cores sensitivities of 95% for ER, 100% for PR, 98% for HER-2 (IHC) and 100% after adding CISH were reached.
Figure 2. A and B. Proportions of immunohistochemically PR-positive and ER-positive cells from all malignant cells in different core samples. Dashed line indicates the cut-off value of 10% for positive and negative cases.
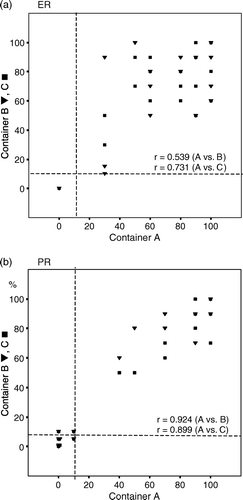
Table III. Sensitivity (%) of different containers and container combinations for the assessment of ER, PR and HER-2.
Discussion
With concordance of 83% for ER, 88% for PR, 88% for HER-2 (IHC) and 93% for HER-2 after adding CISH, our results showed good correlation with assessments from core biopsies of those from surgical specimens. Detailed preoperative histopathologic information is useful for patients who would benefit from neoadjuvat chemotherapy and also for prognostic purposes. There are a few studies comparing the assessment of prognostic factors of core samples to surgical specimens in the literature the results of which are in line with the results of this study (). The heterogeneous expression of prognostic factors in tumour tissue has, however, been a speculative confounding factor in the estimation of cores Citation[3], Citation[5] and therefore the surgical sample has been the gold standard in assessment of ER, PR and HER-2.
Table IV. Agreement and disagreement for ER, PR and HER-2 between core biopsies and surgical specimens. Review of literature.
ER
A study of 51 core samples and subsequent surgical specimens reported that on the surgical specimen ER staining was less profound in the centre than on the edges of the tumour. The same tendency was not noted on the microscopy of cores. This might be due to either homogenous fixation of the cores, whereas the fixation in the centre of the tumour may remain less intense, or a higher chance of sampling the peripheral part of a tumour using core biopsy Citation[4]. In our study the first needle pass that was always targeted to the centre of the tumour did not differ in terms of sensitivity from the more peripheral needle passes for ER expression. This may be due to small tumour size (12 mm, range 5–27 mm) in our study which enables rather homogenous fixation also for the surgical samples. In addition, immunoreactivity for both ER and PR was significantly higher in core biopsies than in surgical samples in our study, which may reflect the better preservation of the antigens by rapid fixation of the cores. In the comparison of proportions of positive ER staining, some heterogeneity was noted between individual cores (). When comparing values dichotomized as positive or negative, the three different containers were concordant in all cases for ER.
PR
In the comparison of proportions of positive staining for PR, heterogeneity between different cores was even less obvious than those for ER. However, for PR, there were six discordant cases between containers, a majority (4) of which were near the chosen cut off value of ≥10%. The commonly used cut-off points of 10% to 20% for positive and negative ER and PR assessments are somewhat arbitrarily selected. Harvey et al. detected that patients with as few as 1–10% weakly ER-positive cancer cells led to significantly improved response to endocrine therapy Citation[18].
HER-2
Tumours with HER-2 overexpression or amplification have been noted to respond to trastuzumab therapy. Lately, it has been increasingly used as an adjuvant treatment for early breast cancer Citation[10–12]. Reliable HER-2 assessment is crucial in order to select the real HER-2 positive patients to receive this possibly life saving therapy and not to unnecessarily expose non-responders (HER-2 negative patients) to potentially serious adverse effects Citation[10], Citation[11].
On IHC there is a tendency for false positive HER-2 assessments, especially with 2+ results Citation[19] depending on the antibody used. Therefore, it is recommended to confirm ICH 2+ and 3+ results Citation[20] for gene amplification either by fluorescence in situ hybridisation (FISH) or with CISH, with an excellent concordance detected between the two tests Citation[8]. HER-2 overexpression or amplification is detected in 9 to 30% of breast cancers Citation[9]. According to our results HER-2 immunopositivity (3 + ) was detected in 24% of the core biopsies; after CISH-confirmation HER-2 gene amplification proved to be present in 15% of the cases, results similar to the other reports.
Core samples seem to be at least as sensitive as surgical specimen in the assessment of ER, PR and HER-2. Similar trend has been detected by some other authors Citation[5], Citation[13–16] (). In the comparison of core samples and surgical specimens, false negative rates for surgical specimens were 14% in the assessment of ER, 15% in the assessment of PR and 50% in the assessment of HER-2 by CISH. Gene amplification was detected by CISH in six core samples, whereas only three surgical samples showed gene amplification. Thus 50% of HER-2 positive cases would have remained undetected if HER-2 assessment by CISH would have been performed from surgical samples only as is the routine in most centres. For core biopsies false negative rates were lower (6%, 0% and 0%, respectively). However, the small number of invasive cancers decreases the statistical power of this study.
In this study most of the tumours were detected at screening mammography and were rather small in comparison with previous studies Citation[13–16]. With large tumours, false negative rates might be even more pronounced because fixation may remain poorer in bigger tumours.
Some heterogeneity between different core samples was detected for ER, PR and HER-2. When dichotomised as positive or negative, different cores proved to be concordant for ER but showed some discordance for PR and HER-2. However, with at least three cores (containers A and B) sensitivity of 100% was reached for HER-2 after adding CISH and with more than three cores (containers A, B and C, the mean number of cores obtained was 7) sensitivities of 100% for PR and, 95% for ER were reached. Thus, in ER-negative cases, the receptor status should be confirmed from the surgical sample. The results are similar with previous studies that determined the amount of core biopsies needed for a reliable histological diagnosis of non-palpable breast lesions Citation[21].
Limitations of the study
The main limitation of our study is the small number of invasive cancers in the study. Because of our intention to investigate tumour heterogenity and to define the number of cores needed for reliable ER, PR and HER-assessments, only cases with invasive cancer both in at least two containers and in surgical specimen were included. Larger studies are needed to confirm the results.
The number of cores obtained was not standardised in our study. If the number was standardised and cores were collected in separate containers each, it would have been possible to determine the minimum number of cores needed for reliable ER, PR and HER-2 assessments. Accordingly, evaluation of heterogeity of ER, PR and HER-2 in tumour tissue would have been more accurate.
Both core samples in different containers and surgical samples were considered sufficient by an experienced pathologist. However, the number of invasive tumour cells in core samples and in surgical specimen should have been counted and a threshold for unambiguous number should have been determined to avoid the possibility of the assessments being based on relatively few cancer cells.
One limitation of our study is also that the study was performed before vacuum-assisted biopsy device was introduced in clinical practice at our hospital. It would be important to know if similar results to 14-gauce core biopsies could be attained using vacuum assisted biopsies with larger sample size.
To conclude, core samples seem to be at least as sensitive as surgical specimen in the assessment of ER, PR and HER-2. According to our results, ER, PR and HER-2 assessments should be considered to be performed from core samples to avoid false negative results and thus not to unnecessarily withhold possibly life-saving therapy from the patient. Some heterogeneity in expression of these factors exists. By obtaining more than three core samples, sensitivity of 100% for PR and HER-2 after adding CISH, 98% for HER-2 (IHC) and 95% for ER can be reached. For additional assurance, negative ER-assessments from core biopsies should be reassessed from surgical samples. Larger studies are needed to confirm these results.
Acknowledgements
This work was supported by grants from Kuopio University Hospital, Paavo Koistinen Foundation, Finnish Breast Cancer Group, Radiological Society of Finland, Kuopio University Hospital Research Foundation, Foundation of Kuopio University, The Ida Montin Foundation, Maud Kuistila Memorial Foundation, Finnish Cultural Foundation of Northern Savo, The Cancer Society of Northern-Savo, Kuopio University and Finnish Cancer Organisations.
References
- Parker SH, Lovin JD, Jobe WE, Luethke JM, Hopper KD, Yakes WF, et al. Stereotactic breast biopsy with a biopsy gun. Radiology 1990; 176: 741–7
- Verkooijen HM, Peeters PH, Buskens E, Koot VC, Borel Rinkes IH, Mali WP, et al. Diagnostic accuracy of large-core needle biopsy for nonpalpable breast disease: A meta-analysis. Br J Cancer 2000; 82: 1017–21
- Denley H, Pinder SE, Elston CW, Lee AH, Ellis IO. Preoperative assessment of prognostic factors in breast cancer. J Clin Pathol 2001; 54: 20–4
- Douglas-Jones AG, Collett N, Morgan JM, Jasani B. Comparison of core oestrogen receptor (ER) assay with excised tumour: Intratumoral distribution of ER in breast carcinoma. J Clin Pathol 2001; 54: 951–5
- Mueller-Holzner E, Fink V, Frede T, Marth C. Immunohistochemical determination of HER2 expression in breast cancer from core biopsy specimens: A reliable predictor of HER2 status of the whole tumor. Breast Cancer Res Treat 2001; 69: 13–9
- Lake DE, Hudis C. Aromatase inhibitors in breast cancer: An update. Cancer Control. 2002; 9: 490–8
- Coleman RE. Current and future status of adjuvant therapy for breast cancer. Cancer 2003; 97: 880–6
- Bhargava R, Lal P, Chen B. Chromogenic in situ hybridization for the detection of HER-2/neu gene amplification in breast cancer with an emphasis on tumors with borderline and low-level amplification: Does it measure up to fluorescence in situ hybridization?. Am J Clin Pathol 2005; 123: 237–43
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–82
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–84
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809–20
- Zidan A, Christie Brown JS, Peston D, Shousha S. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol 1997; 50: 27–9
- Connor CS, Tawfik OW, Joyce AJ, Davis MK, Mayo MS, Jewell WR. A comparison of prognostic tumor markers obtained on image-guided breast biopsies and final surgical specimens. Am J Surg 2002; 184: 322–4
- Badoual C, Maruani A, Ghorra C, Lebas P, Avigdor S, Michenet P. Pathological prognostic factors of invasive breast carcinoma in ultrasound-guided large core biopsies-correlation with subsequent surgical excisions. Breast 2005; 14: 22–7
- Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol 2005; 23: 5148–54
- Becker L, Taves D, McCurdy L, Muscedere G, Karlik S, Ward S. Stereotactic core biopsy of breast microcalcifications: Comparison of film versus digital mammography, both using an add-on unit. AJR Am J Roentgenol 2001; 177: 1451–7
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999; 17: 1474–81
- Bankfalvi A, Simon R, Brandt B, Burger H, Vollmer I, Dockhorn-Dworniczak B, et al. Comparative methodological analysis of erbB-2/HER-2 gene dosage, chromosomal copy number and protein overexpression in breast carcinoma tissues for diagnostic use. Histopathology 2000; 37: 411–9
- Bilous M, Dowsett M, Hanna W, Isola J, Lebeau A, Moreno A, et al. Current perspectives on HER2 testing: A review of national testing guidelines. Mod Pathol 2003; 16: 173–82
- Liberman L, Dershaw DD, Rosen PP, Abramson AF, Deutch BM, Hann LE. Stereotaxic 14-gauge breast biopsy: How many core biopsy specimens are needed?. Radiology 1994; 192: 793–5
Immunohistochemistry (IHC)
The slides were pre-treated in a microwave oven in ChemMate Target Retrieval Solution (dilution 1:10, DAKO, Glostrup, Denmark). Antibody M3569 (DAKO, dilution 1:50) was used to label progesterone receptors, NCL-ER-6F11 (Novocastra, Newcastle upon Tyne, UK, dilution 1:100) to label oestrogen receptors and NCL-CB11 (Novocastra, dilution 1:100) to label HER-2 antigens. The slides were stained in a Techmate 500 Plus automat (DAKO) using the labelled streptavidin biotin method. Peroxidase was the marker enzyme, which was visualised with hydrogen peroxide as a substrate and diaminobenzidine as a chromogen. HER-2, ER and PR positive breast carcinomas were used as positive controls. Substitution of NHS-PBS for the primary antibody solution provided a negative control for each antibody.
Chromogenic in situ hybridisation (CISH)
Spot-Light CISH kit (Zymed 84-0146, Zymed Inc., South San Francisco, CA, USA) was used to detect HER-2 gene amplification. The sections were deparaffinised and incubated in pretreatment buffer in a microwave oven at 93°C for 15 minutes. Slides were digested by enzyme pretreatment reagent (6 to 7 min at room temperature), washed with Aqua and dehydrated with graded ethanols. Ready to-use digoxigenin-labeled HER-2/neu probe of 25–30 µl (consisting of two contiguous BAC clones; Zymed) was applied and the slides were denatured (94°C for 5 min) and hybridised (38–40°C for 17 h). After standard saline and PBS/Tween washes HER-2/neu probe was detected with sequential incubations with antidigoxigenin fluorescein, anti-fluorescein peroxidase, and diaminobenzidine. Sections were lightly counterstained with Mayer's haematoxylin and embedded.