Abstract
Metronomic low-dose chemotherapy regimen was found to have an antiangiogenic effect in tumors. However, its effect on levels of circulating pro-angiogenic and anti-angiogenic factors is not fully explored. Materials and methods. The levels of both VEGF and PDGF-BB were measured in three time points, in the serum of 32 rectal carcinoma patients receiving daily reduced-dose/continuous capecitabine in combination with preoperative pelvic irradiation. Results. We found a significant decrease in VEGF and PDGF-BB serum levels during the combination treatment (p<0.0001), followed by an increase in the successive rest-period (p<0.0001). In addition, substantial changes in platelets counts were observed during treatment in correlation with the changes of VEGF and PDGF-BB serum levels. Discussion. These results suggest that combined chemo-irradiation affect levels of pro-angiogenic factors during treatment, and may reflect an anti-angiogenic window induced during this treatment. The potential implications of this inducible phenomenon, including a possible clinical benefit from the administration of long lasting metronomic chemotherapy immediately following combined chemo-irradiation, would warrant further investigation.
There is a growing body of evidence that lowering the levels of circulating molecules of vascular endothelial growth factor (VEGF) in cancer patients, can improve results of chemotherapy. This has already been demonstrated by studies with humanized monoclonal antibodies to VEGF (Bevacizumab) in patients with metastatic colorectal cancer Citation[1], non small cell lung cancer Citation[2], and ovarian carcinoma Citation[3].
This clinical experience would support the investigation of any additional mechanism that can influence the levels of circulating VEGF. Accordingly, there is evidence from several reports, that the levels of circulating molecules of VEGF in cancer patients can indirectly be affected by conventionally applied treatments. Chemotherapy has been reported to induce depletion of serum-VEGF (sVEGF) levels, although this was significantly more evident in patients who responded to treatment. This phenomenon has been proposed by several investigators as an early predictor of response, probably reflecting reduction in tumor mass Citation[4–6]. Radiotherapy also may affect the levels of sVEGF. However, there is disagreement between increased levels of sVEGF following exposure to ionizing radiation in an experimental model Citation[7], and reduced levels of both sVEGF and sFGF-2 in a clinical study similarly conducted after radiotherapy Citation[8]. Combined chemo-irradiation, as administered in the neo-adjuvant setting for various cancer diseases, has produced conflicting effects on sVEGF levels. In a study on patients with esophageal cancer, sVEGF levels were unaffected by two cycles of chemotherapy administered during weeks one and six along the course of irradiation. Each cycle consisted on five sequential days of fluorouracil followed by cisplatin on day seven Citation[9]. In contrast, a study on patients with head and neck squamous cell cancer revealed that sVEGF levels decreased in direct correlation with better outcome. In that study, 18/25 patients were treated with three cycles of chemotherapy. Each cycle involved five days of continuous infusion of paclitaxel, and was administered every three weeks along the course of irradiation Citation[10].
Platelet derived growth factor (PDGF) is another growth factor with a central role to support tumor vasculature. Accordingly, it has been shown in experimental studies, that the simultaneous inhibition of signaling pathways of VEGF receptors (expressed on endothelial cells) Citation[11] and of PDGF-B receptors (expressed on pericytes) Citation[12], was accompanied by greater effects against tumors, even in late-stage disease Citation[13]. However, up to date there is no study about changes in levels of either sVEGF or sPDGF-BB (the ligand activating PDGF-B receptors), following concomitant treatment by daily/continuous chemotherapy and radiotherapy, in patients with any cancer disease.
We therefore conducted this study to elucidate any possible fluctuations in the levels of these two pro-angiogenic growth factors in the serum, in a series of patients with rectal carcinoma, first as a by-product of neo-adjuvant treatment by irradiation combined with daily reduced-dose/continuous capecitabine, and later as a result of a rest-period with no treatment, as usually allowed in clinical practice.
Materials and methods
Patients characteristics
Thirty two untreated patients with rectal adenocarcinoma were recruited in a single institution between May 2003 and July 2004, after giving their informed consent for participation in the present study which was carried out with ethical committee approval. This work is a correlative study, along a separate clinical study that was conducted in that institute for evaluating the effect of neo-adjuvant radiotherapy to the pelvis combined with continuous oral chemotherapy by capecitabine. The patients' demographics, as well as their tumor characteristics, and their clinical results of treatment, are detailed in a separate paper Citation[14]. Our study population included those 32 of their 43 patients from whom we could obtain all the programmed serum samples. The pre-treatment clinical staging of our patients included loco-regional disease only, with T3/T4 rectal tumors and various N categories (in 30/32), or T2N0 tumors located <5 cm from the anal verge (in 2/32). Radiotherapy was delivered to the posterior pelvis, to a total dose of 45 Gy in 25 fractions over five weeks, with an additional boost of 5.4 Gy in three fractions limited to the tumor area.
Patients received capecitabine orally (825 mg/m2 twice daily) for five days a week, concomitantly with radiotherapy. A rest-period took place thereafter, with no further treatment until surgery.
Response to treatment was assessed by comparing the post-treatment pathological stage to the pre-treatment clinical stage, and the results were as follows: Complete Response-30%; Microscopic Residual-12%; Partial Response-37% (defined as the reduction of Tumor or Lymph Node stage or both); No Response-21%.
Evaluation of sVEGF, sPDGF-BB and blood cells
Blood samples were collected from each participant in the study, at three predetermined time-points (T.P.s): T.P.1.: before any treatment; T.P.2.: immediately by the end of treatment (chemo-irradiation); T.P.3.: following a median rest-period of five weeks (range four to six weeks). Each blood sampling (12 milliliters) served for determining the levels of sVEGF and sPDGF-BB (10 milliliters) and differential blood counts (two milliliters). The first were collected in serum separator tubes (SST) and allowed to clot for 30 minutes, followed by centrifugation at room temperature for 15 minutes at 1000 g. The separated serum was removed and divided in two separate ependorfs and stored at −20°C. sVEGF and sPDGF-BB levels were later determined by the Enzyme-Linked immunosorbent assay (ELISA) using Quantikine R&D system kits, following the manufacturer's instructions.
Results of both sVEGF and sPDGF-BB levels were expressed as pg/ml, and calculated as mean values +/− standard deviation (S.D.) (). Complete blood counts were analyzed in parallel to each measurement of sVEGF and sPDGF-BB. The consecutive mean (S.D.) counts of each cellular component of blood were studied in correlation with the corresponding consecutive mean (S.D.) levels of each growth factor.
Table I. Levels of sVEGF, sPDGF-BB, and PLT counts, at three consecutive time-points (T.P.s).
Statistical analysis
Statistical evaluations were conducted using the SPSS statistical package. We used ANOVA with repeated measures, and Bonferroni adjustment for multiple comparisons, to compare the levels of the study variables (sVEGF, sPDGF-BB and various cellular components of blood) in three time-points in the whole patients' group. A comparison of the percentage of decreases in levels of sVEGF and sPDGF-BB between four independent sub groups of patients, as classified according to the various degrees of response, was done by Kruskal-Wallis Test, because of small sub groups. Statistical significance was set at a p-value <0.05. All statistical tests were two-sided.
Results
Among 32 patients with rectal carcinoma receiving combined chemo-irradiation, the serum levels of both pro-angiogenic factors, VEGF and PDGF-BB, showed consistent and statistically highly significant changes (p < 0.0001), between each two consecutive measurements (, and ).
Figure 1. Serum levels of VEGF at three time-points: 1. before chemo-irradiation; 2. at the end of chemo-irradiation; 3. following a rest-period with no treatment; Value of mean is indicated in the graph at each time point.
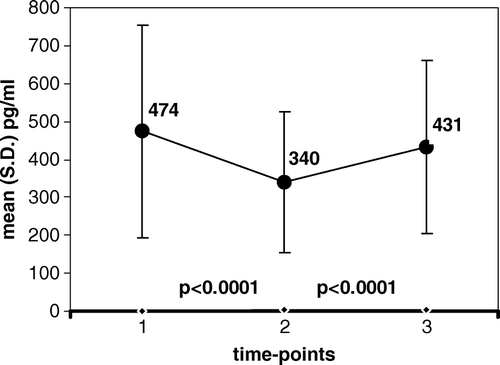
Figure 2. Serum levels of PDGF-BB at three time-points: 1. before chemo-irradiation; 2. at the end of chemo-irradiation; 3. following a rest-period with no treatment; Value of mean is indicated in the graph at each time point.
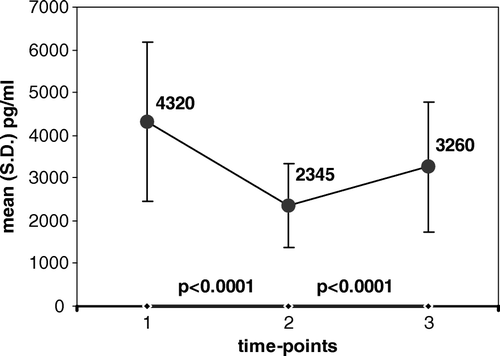
The mean (S.D.) levels of sVEGF measured before any treatment (T.P.1.) decreased by the last day of pelvic irradiation (T.P.2.) in 28/32 patients, and increased in only 2/32 patients, resulting in significantly reduced values, from 474 (279) to 340 (186), (p < 0.0001). Similarly, the values of sPDGF-BB decreased by the end of treatment in 31/32 patients and increased in only1/32 patient, resulting in significantly reduced values, from 4 320 (1 863) to 2 345 (978), (p < 0.0001).
A significant increment in the levels of both pro-angiogenic factors later developed, between the end of chemo-irradiation (T.P.2.) and their repeated measurement following a rest-period and still before surgery (T.P.3.). Levels of sVEGF increased during that period in 24/32 patients and remained stable (with less than 10% change) in 7/32 patients. The resulting increment of sVEGF from 340 (186) to 431 (228) was statistically significant (p < 0.0001). At the same time, also the values of sPDGF-BB significantly increased in most patients (28/32), while decreasing in only 3/32 patients. This resulted in significantly increased values in the interval, from 2 345 (978) to 3 284 (1 525), (p < 0.0001).
Counts of all cellular components of blood were obtained at all three determined time-points from 27 of 32 patients receiving neo-adjuvant chemo-irradiation. Only platelets (PLT) counts showed significant change following treatment. The mean PLT counts decreased from 318×103 (120×103) before treatment (T.P.1.) to 241×103 (66×103) at the end of treatment (T.P.2.), (p = 0.05).
This drop in PLT counts during chemo-irradiation was in accordance with the drop in levels of both sVEGF and sPDGF-BB, although it was statistically less significant (p = 0.05 vs. p < 0.0001). However, during the subsequent rest-period, changes in PLT counts were non-significant at all, as opposed to the significant increment (p < 0.0001) of both sVEGF and sPDGF during the same period.
A separate analysis of the changes in levels of both sVEGF and sPDGF-BB following chemo-irradiation was performed in correlation with different types of response to treatment. We found no significant difference in the percentage of decrease in serum levels between the corresponding subgroups of patients, including complete responders compared with non-responders at all (p = 0.454 for sVEGF, and p = 0.531 for sPDGF-BB).
Discussion
This study is the first to document consistent and significant reduction of both VEGF and PDGF-BB levels in the serum in patients receiving combined treatment by chemo-irradiation. Furthermore, it shows that this effect is transient in most participants for both pro-angiogenic factors, as manifested by a significant degree of reconstituted levels when these were measured after a median rest-period of five weeks (range four to six). We discovered similar changes in the levels of both pro-angiogenic factors in the serum, both following chemo-irradiation and following a rest period. This may suggest an epi-angiogenic phenomenon in response to combined treatment by chemo-irradiation.
Although absolute values of circulating VEGF and PDGF-BB are better determined by their measurement in the plasma (rather than in the serum), there is enough evidence that changes in the levels of circulating VEGF and PDGF-BB can similarly be determined in both serum and plasma Citation[15]. Therefore, our results of repeated measurements of VEGF and PDGF-BB levels in the serum are valid and reflect genuine changes during and after treatment, which are also reported by other studies that tested serum levels rather than plasma levels of various pro-angiogenic factors Citation[16].
We asked whether the almost unanimous drop in sVEGF levels following combined chemo-irradiation (by more than 10% in 28/32 patients), reaching high statistical significance for the whole group (p < 0.0001), was mainly induced by radiotherapy or by the chemotherapy added to irradiation. In order to answer that question we had to compare our results with those from similar measurements in subjects treated for rectal carcinoma by either irradiation to the pelvis without concomitant chemotherapy or by metronomic chemotherapy alone. However, all our rectal cancer patients were treated by pelvic irradiation with concomitant chemotherapy and we could not obtain an appropriate control group. Also the available data in the literature is insufficient for definitively answering that question, since no report was found on changes in levels of circulating VEGF (or PDGF-BB) in CRC patients following isolated pelvic irradiation, or following solely metronomic chemotherapy.
We found one study reporting that sVEGF levels significantly decrease after anti-neoplastic radiotherapy Citation[8], apparently in agreement with the decrease following combined chemo-irradiation in our series. This agreement between results of the two studies, would suggest that irradiation did play a role in the decrease of sVEGF levels. However, this does not eliminate the possibility that continuous chemotherapy also contributed to the most significant drop of serum levels of both sVEGF and sPDGF-BB recorded in our study. In fact, the extent of diminution of both factors following chemo-irradiation was almost universal in our series, as opposed to its dependency both on response and on the dose of radiation, as found by the other investigators.
Although changes in pro-angiogenic factors may be affected by changes in tumor mass in patients responding to treatment, our results may suggest an alternative explanation, since no correlation was observed between the different types of response to chemo-radiation and the mostly uniform and significant incidence and magnitude of decrease in the levels of both growth factors in the serum. The decrease was similar in the 42% patients with best response (30% with complete response and 12% with only microscopic residual), and in the 37% patients showing partial response, and in the 21% patients with no response at all: p = 0.454 for sVEGF, and p = 0.531 for sPDGF-BB. Thus these results may suggest, that the changes in levels of sVEGF and sPDGF-BB are at least in part a treatment related effect, and not only a tumor mass effect in response to treatment.
The probability that daily low-dose/continuous capecitabine did contribute to the reduction of circulating levels of the pro-angiogenic factors is supported by another study on low-dose and continuous (metronomic) chemotherapy, although that study differed from ours with regards to both the disease and the drugs under investigation, as it was conducted on breast cancer patients treated by oral cyclophosphamide and methotrexate Citation[16]. That study showed that sVEGF levels progressively decreased in direct correlation with the length of treatment persistence. In agreement with our study, this occurred irrespectively of tumor response. The absence of that correlation would imply that while the suppression of pro-angiogenic factors by metronomic chemotherapy treatment may contribute to the induction of clinical benefit, it is not always sufficient for achieving that degree of benefit.
Our findings regarding sPDGF-BB, which were in parallel with those regarding sVEGF, could not be discussed in comparison with other studies, in view of the lack of relevant data in the literature.
Another significant treatment-related change in the serum-levels of the two pro-angiogenic factors was demonstrated in our study, consistent in their increment during the four to six weeks of rest-period following chemo-irradiation. This appeared to represent a process of “recovery” towards pre-treatment levels, taking place as soon as chemo-irradiation was stopped. We could find only two relevant studies in the literature Citation[9], Citation[10]; both were limited to sVEGF, and their conclusions were in disagreement with ours. However, we suggest that both of them might have inadvertently missed the process of increment in sVEGF levels during that interval, because of their different study design. In both of them, only one post-treatment measurement of sVEGF was performed, and this was done at non-uniform time-points, aimed at evaluating post-treatment levels in comparison with pre-treatment levels, without reference to the time factor. We, on the other hand, performed two measurements at the post-treatment period and both were carried out at uniformly predetermined time-points, thus discovering the significant increment that took place during the rest-period.
What could be the biological mechanism underlying the drop in levels of both sVEGF and sPDGF-BB following combined daily reduced-dose /continuous capecitabine and irradiation in our study, as well as their increment following the discontinuation of treatment? The repeated counting of all blood cells at a uniform schedule of time-points, in parallel with the measurements of the two growth factors, revealed that the changes in their levels following chemo-irradiation were in direct correlation with changes in platelet (PLT) counts (). This is in line with the function of PLTs as transporters of VEGF in the circulation Citation[17] and with the high content of both VEGF Citation[18] and PDGF-BB Citation[19] within PLTs. Correspondingly, and supporting our findings, a direct correlation has been demonstrated, including in patients with colorectal cancer, between number of PLTs and levels of VEGF in the serum Citation[20]. This correlation is supported also by an observation made on a series of patients with breast cancer Citation[17], where the investigators noticed that after an initial episode of thrombocytopenia, consequent to chemotherapy with doxorubicin and cyclophosphamide at MTD schedule, there was a strong platelet rebound that coincided closely with a serum VEGF peak (p < 0.01). However, in our study this correlation was not absolute. We found that although the reduction of sVEGF (and of sPDGF-BB) levels by the end of chemo-irradiation (p < 0.0001) did correlate with reduction of PLT counts (p = 0.05), there was no similarly significant increment of PLT counts accompanying the increment in serum levels of the growth factors during the subsequent rest-period. This would suggest that although PLT counts may have some role in the changes of sVEGF and sPDGF-BB levels during and following our treatment by daily low-dose/continuous capecitabine, that role is not yet elucidated. In addition, the changes in PLT counts would not represent the only plausible explanation for the changing levels of sVEGF and sPDGF-BB. This is implied from the study of Colleoni Citation[16], who showed that a significant reduction of sVEGF levels (p = 0.001) occurred in patients treated by continuous low-dose oral cyclophosphamide and methotrexate although this was not accompanied by changes in platelet counts.
It has been shown in experimental studies that the anti-tumor effect of metronomic chemotherapy is accompanied by increment of the angiogenic inhibitor TSP-1 both in the circulation Citation[21] and in the tumor tissue Citation[22]. It may thus be possible that up-regulation of TSP-1, is one of the anti-angiogenic properties of metronomic chemotherapy. By the same line, it may be possible that in our study the down-regulation of VEGF and PDGF-BB was either a consequence of metronomic chemotherapy or a new phenomenon which provides another explanation for the anti-angiogenic property attributed to metronomic chemotherapy.
In summary, we conclude that circulating levels of both VEGF and PDGF-BB significantly decrease during treatment by daily low-dose/continuous capecitabine combined with neo-adjuvant irradiation in rectal carcinoma patients (p < 0.0001), and significantly increase during a successive rest-period (p < 0.0001). These findings, which are most probably induced by treatment procedures, may represent a so far disregarded anti-angiogenic window. Their potential implications, including a possible benefit from the administration of long lasting metronomic chemotherapy immediately following treatment by combined chemo-irradiation, would warrant further investigation.
Conflict of interest
The authors indicated no potential conflicts of interest.
Acknowledgements
We thank RN Batia Miller and RN Ziona Sadeh for their significant assistance in the conduction of the study.
References
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwrigt T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–50
- Garcia AA, Oza AM, Hirte H, Fleming G, Tsao-Wei D, Roman L, et al Interim report of a phase II clinical trial of bevacizumab (Bev) and low dose metronomic oral cyclophosphamide (mCTX) in recurrent avarian (OC) and primary peritoneal carcinoma: A California Cancer Consortium Trial. J Clin Oncol 2005;23; 455s Abstract 5000.
- Lissoni P, Fugamalli E, Malugani F, Ardizzoia A, Secondino S, Tancini G, et al. Chemotherapy and angiogenesis in advanced cancer: Vascular endothelial growth factor (VEGF) decline as predictor of disease control during taxol therapy in metastatic breast cancer. Int J Biol Markers 2000; 15: 308–11
- Mahaseth H, Dudek AZ. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: Correlation with treatment response and survival. Cancer Invest 2005; 23: 193–200
- Pedersen LM, Klausen TW, Davidsen UH, Johnsen HE. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin's lymphoma. Ann Hematol 2005; 84: 510–6
- Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 1999; 59: 3374–8
- Ria R, Portaluri M, Russo F, Cirulli T, Di Pietro G, Bambace S, et al. Serum levels of angiogenic cytokines decrease after antineoplastic radiotherapy. Cancer Lett 2004; 216: 103–7
- McDonnell CO, Harmey JH, Bouchier-Hayes DJ, Walsh TN. Effect of multimodality therapy on circulating vascular endothelial growth factor levels in patients with oesophageal cancer. Br J Surg 2001; 88: 1105–9
- Druzgal CH, Chen Z, Yeh NT, Thomas GR, Ondrey FG, Duffey DC, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck 2005; 27: 771–84
- Dvorak HF. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A critical cytokine of tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–80
- Sundberg C, Ljungstrom M, Lindmark G, Gerdin B, Rubin K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol 1993; 143: 1377–88
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 2003; 111: 1287–95
- Yerushalmi R, Idelevich E, Dror Y, Stemmer SM, Figer A, Sulkes A, et al. Preoperative chemoradiation in rectal cancer: Retrospective comparison between capecitabine and continuous infusion of 5-Fluorouracil (5-FU). J Surg Oncol 2006; 93: 529–33
- Dudek AZ, Mahaseth H. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: Correlation with treatment response and survival. Cancer Invest 2005; 23: 193–200
- Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole’ F, et al. A Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: Antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002; 13: 73–80
- Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, et al. Platelet: Transporter of vascular endothelial growth factor. Clin Cancer Res 1997; 32: 187–90
- Salgado R, Benoy I, Bogers J, Weitjens R, Vermeulen P, Dirix L, et al. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study. Angiogenesis 2001; 4: 37–43
- Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg 2004; 114: 1502–8
- George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: Clinical evidence of platelet scavenging?. Clin Cancer Res 2000; 6: 3147–52
- Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin-1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA 2003; 100: 12917–22
- Damber JE, Valbo C, Albertsson P, Lennernas B, Norrby K. The antitumor effect of low-dose continuous chemotherapy may partly be mediated by throbospondin. Cancer Chemother Pharmacol 2005; 7: 1–7