Abstract
Background. Randomized controlled trials and service screening programs have shown that breast cancer screening reduces the mortality from the disease. Several years of monitoring are needed to prove such an effect. In the meantime attention should be paid to early surrogate measures, such as histopathological tumor characteristics. The Norwegian Breast Cancer Screening Program started November 1995. This study compares incidence, prognostic tumor characteristics and surgical treatment in breast cancer cases diagnosed in the pre-screening (1987–1995, n=2 618) and screening period (1996–2004, n=5 417), in women aged 50–69 years, residing in the first four counties implementing the screening program. The screening period is divided into those invited versus those not invited to the screening program, and those exposed (participants) versus those not exposed to the program (non-participants). Results. The incidence of invasive breast cancer rose from 170 per 100 000 women years (wy) in 1987 to 355 per 100 000 wy in 1997. The proportion of DCIS was 5% in the pre-screening period, and 14% in the screening period. Tumors 20 mm or less were diagnosed in 56% of the invasive cases in the pre-screening period, in 74% of the invited, and in 77% of the exposed women. The relative risk of diagnosing breast cancer with metastases was 0.85 (95% CI 0.84–0.87) for invited and 0.82 (95% CI 0.81–0.84) for exposed women, relative to those diagnosed in the pre-screening period. Ablation was performed in 85% of the invasive cases diagnosed in the pre-screening period, and in 45% of the cases in the screening period. Conclusion. Breast cancer diagnosed in the screening period had prognostically favorable tumor characteristics compared to breast cancer diagnosed in the pre-screening period. Implementation of organized population based screening and the time trend are considered possible reasons.
Randomized controlled trials (RCT) and service screening programs with mammography have demonstrated a substantial reduction in breast cancer mortality in women invited to screening Citation[1], Citation[2]. The evaluations have shown that 5–10 years of follow-up is needed for the mortality results to emerge Citation[1], Citation[2], because there is a relatively high survival rate from the disease Citation[3]. In the mean time, attention must be paid to early surrogate measures Citation[4], Citation[5], such as the prognostic factors based on histopatological tumor features, which are expected to be prognostic favorable in women exposed to screening as compared to those not exposed to screening Citation[4], Citation[5]. The efficacy of a screening program is usually estimated in the view of “intention to treat”. However, it is of over all interest to evaluate the prognostic factors both among the women invited, as well as among those who actually participated and had a breast cancer diagnosed as a result of adherence to the screening program.
The Norwegian Breast Cancer Screening Program (NBCSP) started in four of 19 counties in Norway in 1996 (Akershus, Hordaland, Oslo and Rogaland). The program gradually expanded and became nationwide in 2004. About half a million women aged 50–69 years are biennially invited to a two view mammography of 2007 Citation[6], Citation[7]. Due to the recent nationwide coverage, the program is in the phase of evaluating performance and early surrogate measures, which can be considered as an alternative to, and preparation for analyzing the mortality Citation[5]. Evaluation of early surrogate measures refers mainly to comparisons of achieved results with recommended and desirable levels given in guidelines Citation[5]. The guidelines are based on the experiences from the randomized trials and screening programs. However, comparing the indicators, such as the proportion of invasive tumors with regional or distant metastases (advanced tumors) in cases diagnosed before and after implementation of a screening program can also be considered of great value to identify the extent of the stage shift that has taken place. Stage shift is one of the assumptions for a mortality reduction as a result of the screening Citation[4], Citation[5].
We decided to take advantage of the information collected as a part of the registration of the cancer cases at the Cancer Registry of Norway, and in the screening program. The incidence of invasive cases, the histological type, the histopathological tumor size and the presence of metastases, in addition to the surgical treatment in breast cancer cases diagnosed in the pre-screening (1987–1995) and screening period (1996–2004) are thus compared.
Material and methods
This study includes women residing in Akershus, Hordaland, Oslo and Rogaland who were aged 50–69 years at diagnosis of the first breast cancer, which was in 1987–1995 (pre-screening period) or 1996–2004 (screening period). The women diagnosed with cancer during the screening period were stratified by a; invited and not invited to the screening program and b; exposed and not exposed to the screening program. All women who were invited into the screening program, and subsequently were diagnosed with breast cancer are in the invited group, regardless of whether or not they participated in the screening. Those not invited were women diagnosed with breast cancer before the first stated date for the screening examination. The term exposed is used to define cancers detected as a result of participation in the screening program, either as a cancer detected at screening (screen detected), or as a cancer detected in the period between two screening sessions after a negative screening outcome (interval breast cancers). Not exposed was defined as breast cancer cases diagnosed before the stated date of screening examination in the screening program, and breast cancer in women who did not participate in the screening program despite being invited. The pre-screening period obviously did not include invited or exposed women.
Breast cancer cases
A total of 8 090 breast malignancies were diagnosed in the study period. Fifty-two cases were excluded due to cancer of other origin or missing information about histological type. Three cases were excluded due to detection in the screening program in 1995 (in Rogaland). The total number of breast cancer cases evaluated was thus 8 035. A total of 2 618 cases (84 DCIS and 2 534 invasive) were diagnosed in the pre-screening period and 5 417 (775 DCIS and 4 642 invasive) in the screening period. Advanced tumors () is defined as invasive tumors with regional or distant metastases, while not advanced tumors are defined as tumors without metastases.
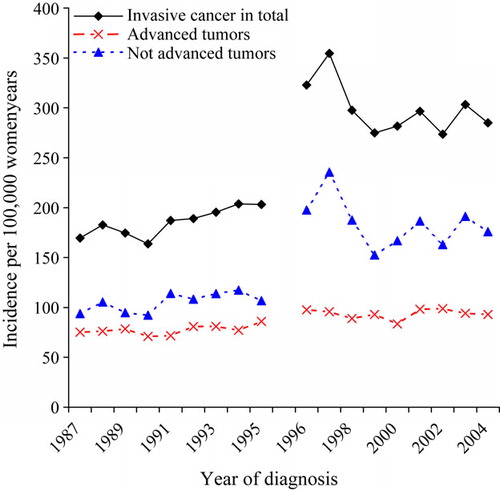
Figure 1. Incidence of invasive breast cancer in total, advanced and not advanced tumors per 100 000 women-years in the pre-screening (1987–1995) and screening period (1996–2004) in Norwegian women aged 50–69 years at diagnosis. Women resident in Akershus, Hordaland, Oslo and Rogaland.
Mean age was 60 years for women diagnosed in the pre-screening period, and 59 for those diagnosed in the screening period. Median age for the groups was 61 and 58 years, respectively for the prescreened and screened group. The invited women were on average 59 years old (median 59 years), and the not invited women were on average 57 years old (median 55 years).
Data source
The data were extracted from the main database at the Cancer Registry of Norway, and from the NBCSP-database, by September 2006. A unique eleven-digit personal identification number given to all inhabitants in Norway was used to merge the two databases. It has been mandatory by law for all physicians and pathology departments to report all cancer cases since 1952, which makes the Registry almost complete for solid tumors Citation[3]. A regulation allows research on cancer data from the Cancer Registry without informed consent from the cancer cases Citation[8]. The reported cases are coded according to the International Classification of Disease (ICD7 or ICD10), and transferred into pathological Tumor Nodule Metastasis (pTNM) classification. The information about tumor size and metastases were based on the pTNM-code. The completeness of the information has increased during the last decades, but unfortunately data are still missing, or not collected. Due to lack of information about DCIS before 1993, the first breast cancer is defined as the first invasive breast cancer before 1993, and the first invasive or DCIS in 1993 and later.
The Norwegian Breast Cancer Screening Program
The screening program started in one county late November 1995 (Rogaland), and in three counties in 1996 (Akershus, Hordaland and Oslo). The NBCSP is described in details elsewhere Citation[6], Citation[7]. In short, the program is an organized population-based screening program inviting all Norwegian women aged 50–69 years, by a personal letter, to a stated place and time for bilateral two-view mammography biennially. The program utilizes independent double reading with consensus. During the first ten years of performance, a total of 1 390 310 invitations were sent whereas 1 059 309 screens were performed (76.2%) Citation[7]. The screening detection rate was 5.5 per 1 000 screens and the interval cancer rate 1.9 per 1 000 screens. A total of 15.4% of the cancers (screen detected and interval cancers) were DCIS.
Statistics
The incidence of breast cancer will be shown as the rate of invasive breast cancer cases diagnosed per 100 000 women years (wy) in total, and separately for advanced and not advanced tumors (). The distributions of histological type, histopathological tumor size, metastases, and surgical treatment will be given as proportions and relative risks (RR). Confidence intervals (CI) and p-values were estimated by χ2, and a p-value equal to or less than 0.05 was regarded as statistically significant. All analyses were conducted using SPSS (SPSS, version 14.0.1 for Windows, SPSS Inc, Chicago, Illinois).
Results
The incidence of invasive breast cancer increased from 170 to 203 per 100 000 wy in the pre-screening period, 1987–1995 (). The rise continued during the screening period, and reached a top with 355 per 100 000 wy in 1997. After this incline, the incidence decreased and stabilized at around 290 per 100 000 wy. The incidence of advanced tumors increased from 75 to 86 per 100 000 wy in the pre-screening period, and then to 98 and 96 per 100 000 wy in 1996 and 1997, which represent the first two years in the screening program. This prevalent peak was followed by an incidence varying from 84 to 99 per 100 000 wy in the period 1998–2005. The rate of not advanced tumors had a similar trend as the total rate, but at a lower level, and with a slower increase in the pre-screening period, from 94 to 107 per 100 000 wy. The marginal increase was replaced by a steep increase up to 236 per 100 000 wy, before a stabilization of the incidence of about 175 per 100 000 wy occurred for the rest of the screening period.
A significantly higher proportion of invasive ductal carcinomas and a lower proportion of lobular cancers were seen in the cases diagnosed in the pre-screening period compared to the screening period (p < 0.001 for both invited and exposed women, ). Invasive tumors 20 mm or less was seen in 55.7% (778/1 396) of the cases diagnosed in the pre-screening period and 73.2% (2 829/3 865) of the cases in the screening period (p < 0.001). This gives a RR of 0.76 (95% CI 0.75–0.78) for having a tumor 20 mm or less in the screening period relative to the pre-screening period. All groups in the screening period had a higher proportion of tumors 20 mm or less relative to the pre-screening period. The numbers in shows a RR of 0.75 (95% CI 0.73–0.77) for having breast cancer 20 mm or less, for invited women, and a RR of 0.87 (95% CI 0.83–0.91) for not invited women, relative to the pre-screening group. Corresponding risks for exposed and not exposed were 0.73 (95% CI 0.71–0.74) and 0.90 (95% CI 0.88–0.93). Only a small fraction of the tumors in all groups were larger than 50 mm or had grown into the chest wall. The largest percentage was seen in the pre-screening group (11.1%, 155/1 396), the lowest in the exposed group (3.1%, 92/2 920, p < 0.001). The tumor size of invasive cancers increased significantly by age only in women diagnosed in the pre-screening period (p = 0.008, data not in Table).
Table I. Number and proportions of invasive breast cancers diagnosed in the pre-screening (1987–1995) and screening period (1996–2004), in women aged 50–69 years resident in Norway*, by type, tumor size, metastases and surgical treatment.
The proportion of advanced tumors was 42.4% in the pre-screening period (1 059/2 495), 30.2% in the group of exposed women (958/3 172, p < 0.001), and 43.7% among the not exposed women (500/1 144, p = 0.498). The relative risk of an advanced tumor was 0.85 (95% CI 0.84–0.87) for invited women, and 0.99 (95% CI 0.97–1.0) for not invited women, relative to the pre-screening group. The risks were 0.82 (95% CI 0.81–0.84) and 1.02 (95% CI 1.01–1.03) for exposed and not exposed women, respectively.
Breast conserving surgery was performed in 15.0% (361/2 403) of the women with invasive cancer diagnosed in the pre-screening period, and 49.1% (1 612/3 282, p < 0.001) of the women exposed to screening. In the groups of not invited and not exposed women, 25.5% (143/561) and 33.8% (391/1 157), had breast conserving surgery. The RR of ablation was thus 0.33 (95% CI 0.31–0.35) for women diagnosed in the screening period, relative to that of the pre-screening period. The risk of ablation was 0.31 (95% CI 0.28–0.33) for exposed and 0.44 (95% CI 0.39–0.49) for not exposed women, relative to that of the pre-screening group.
The proportion of DCIS was 5.1% (49/966) in the pre-screening period 1993–1995, and 14.3% (775/5 417, p < 0.001) in the screening period (not in Table). A total of 15.1% (722/4 779) of the invited and 15.8% (635/4 026) of the exposed women were diagnosed with DCIS. The proportion of DCIS was 8.3% (53/638) in the not invited group and 10.1% (140/1 391) in the not exposed women. Information about surgical treatment was available in 49 DCIS-cases in the pre-screening period, of which 12 cases (24.5%) had breast-conserving surgery. The corresponding number for the screening period was 391 of 775 cases (50.5%).
Discussion
Invasive tumors diagnosed in the screening period (1996–2004) had prognostically favorable histopathological characteristics, and were more likely to be treated with breast conserving surgery compared to those diagnosed in the pre-screening period (1987–1995). The proportion of advanced tumors increased probably as a result of the implementation of the screening program, but the expected decline after the first years of performance is not clearly apparent in this study.
Results from randomized controlled trials have demonstrated that breast cancer is a progressive disease, whose progression can be arrested by early detection in the preclinical phase Citation[1], Citation[2]. These findings have further led to the conclusion that whether the treatment is given early or late in the natural history of the disease is more important for the outcome than the mode of treatment Citation[9]. The goal in breast screening programs is thus to detect the breast cancers as early as possible in its natural progression.
An increase in the incidence of breast cancer is expected just after the initiation of a screening program (a prevalent peak), due to lead time, and detection of both preclinical and clinical cancers Citation[4]. Lead time refers to the time between when a cancer is diagnosed by screening and when it would appear through clinical sign and symptoms. An effective screening program should lead to a decline of advanced tumors among the screened women after the first screen assuming the cancers at the subsequent screens were detected in an early stage Citation[4], Citation[5], Citation[9]. Such a pattern is not clearly apparent in this study, which may be due to several factors, which are also described in a paper focusing on the incidence before and after implementation of organized population based screening in Norway Citation[10]. First, women not invited or not exposed contribute greatly to the proportion of advanced tumors (). Second, screened women in Norway have been frequent ever users of hormonal therapy, which is known to increase the risk of invasive tumors Citation[11], Citation[12]. Furthermore, the introduction of sentinel node diagnostics concurrent with the implementation of the screening program may have increased the detection of positive nodes, because sentinel node technique increases the sensitivity of detecting metastases of breast cancer Citation[13]. Another hypothesis is that too few advanced tumors were diagnosed in the first screening round of the program. If there had been a more substantial increase of advanced tumors during the first round of screening it would have resulted in a higher prevalent peak, and thus at the subsequent rounds there would have been a more pronounced decrease in incidence, assuming similar number of cancer cases detected in the dedicated period.
The lack of reduction in the incidence rate of invasive cancers some years after implementation of the screening program has been hypothesized to be due to overdiagnosis Citation[14]. Overdiagnosis is defined as the detection of cancers that would otherwise not have been diagnosed in the women's lifetime without screening. It is obvious that screening detects slow-developing growing cases which have a long lead-time, and thus survival will be better in cases detected by screening without there necessarily being a real difference between the survival of screened and unscreened populations Citation[4]. This is referred to as length time bias, and is usually considered in a biostatistical and epidemiological approach. Most studies conclude that overdiagnosis mostly pertain to DCIS, but it has also been claimed to occur in invasive cases Citation[14], Citation[15]. The aim of a screening program is to detect the cancer as early as possible in its natural progression as possible. However, the breast cancer biology is heterogeneous, which makes it difficult to predict which tumors progress rapidly versus slowly and some may even go into a steady state Citation[9], Citation[16]. Currently, there are no evidence-based studies that provide data to distinguish between which tumors should, and should not be treated, making it ethically necessary to offer treatment to women diagnosed with all kind of breast cancer. Results from well designed scientific studies suggest that about 3–10% of the breast cancer cases diagnosed in a screening program can be considered a result of overdiagnosis Citation[15], Citation[17]. It is reasonable to assume a similar proportion in this study population.
A higher rate of invasive lobular cancer was shown in the screening period, compared to the pre screening period. This increase and the increase in detection of tumors less than or equal to 20 mm in the screening period, is probably mainly related to the implementation of the screening program, but also because of improved equipment, more experienced radiologists, and increased attention towards breast cancer and mammography in general. A previous study from Norway, where all screen and interval cancers in this study are included, shows close correlation between grading and lymph node involvement with tumor size Citation[7]. The negative correlation between tumor size and survival is well known for invasive cases Citation[9], Citation[18], and a mathematical model by Michaelson et al. shows that the chance of dying from breast cancer increases by 1% for every additional mm of tumor diameter Citation[19].
The proportion of breast cancer cases treated with breast conserving treatment has increased over time, and is the most common surgical treatment today. The increased proportion of this treatment is probably due to smaller tumor size and less invasive tumors with metastases, but also the fact that the technique was shown to have similar survival as mastectomy in the mid 1990's Citation[20], which can be regarded as a change in treatments due to the time trend.
DCIS comprised 5% of the cancers in the pre-screening period, and 15% of the cancers in the screening period, which is indubitably related to the screening program, and the possibility of overdiagnosis. The understanding of the non-invasive forms of ductal carcinoma is evolving. DCIS lesions were rarely diagnosed 20 years ago Citation[21], and studies have shown different aggressiveness in the lesions, and it is thus difficult to predict which case will progress to invasive cancer. In the Two-county trial, DCIS accounted for approximately 12% of the reduction in deaths Citation[22]. Some breast cancers are clinically unimportant because undetected DCIS and invasive breast cancers have been found in autopsy studies Citation[23]. The proportion of DCIS both in the pre-screening and screening period in this study are comparable with results from other studies Citation[4], and follows the recommendations given in the European Guidelines Citation[5]. However, identification and treatment of DCIS emerges as a challenge in screening programs due to its incidence and the lack of evidence for invasion, and thus the possibilities of overtreatment. The proportion of breast conserving treatment in DCIS was 51% in the screening period. Its non-invasive biology, and the similar proportion of breast conservative treatment among the invasive cases made us expect a higher proportion of breast conservative treatment in that group. Recurrence rate of DCIS vary by treatment Citation[24] and multifocality may be the main reason for doing mastectomies instead of breast-conserving treatment.
In this study exposed cases are diagnosed as a result of participation in the NBCSP, while invited cases include both exposed and not exposed women. Exposed cases consist both of screen detected and interval cancers, while not exposed cases are diagnosed as a result of participation in screening at a private clinic, or because of clinical findings. The reason for not attending the NBCSP could be preferences to private screening or no trust in mammography and breast cancer screening, and it is expected that several socioeconomic differences exist in the women who attend and do not attend a population based screening program Citation[25]. In the pre-screening period, all cases were diagnosed clinically or at private screening. Information about mammographic performance at private clinics in Norway is not available today.
This study stratifies the analyses to examine a pre and post screening period and by exposure to the screening program. During the years between the pre-screening and screening periods, breast imaging equipments and staff have improved and may have some bearing on the tumor characteristics, which is a limitation of the study. A control group including cancer cases diagnosed in the same time period in counties not offering organized screening could have been considered to control for the time trend. However, the effect of the gradual expansion of the screening program to include all counties in Norway influenced breast cancer screening throughout Norway and therefore a control group of cancer cases diagnosed in the non screening may also be biased. On the other hand, the exposed and not exposed groups could be considered as cases and controls that were diagnosed in the same county in the same time frame. Detection mode are shown to be an important prognostic factor for breast cancer survival, even after adjusting for known tumor characteristics Citation[24].
The lack of information about DCIS from the pre-screening period is another limitation of this study. Further, the proportion of cases with missing information is somewhat high, and there is a lack of detailed information about histopathologic tumor size and grade. The lack is most pronounced in the pre-screening period, probably because of less knowledge and attention to prognostic tumor characteristics, in the pre screening versus the screening period. The missing data may bias the results, and has to be considered when drawing the conclusion of the study. A further study about the trend of the completeness of reporting by hospitals, and geographic area would be of interest, and a step forward to obtain complete data. The strength of this study is the size of the population studied, complete data on the cancer cases, and the ability to stratify both according to invited and not invited women, but also according to exposed and not exposed.
In conclusion, the histopathological tumor size is reduced and metastases less frequently present in women diagnosed in the screening period compared to those diagnosed in the pre-screening period. Further, breast conservative surgery was more frequently performed in the screening period, compared to the pre-screening period. Our study population represents about 40% of the Norwegian women in the 50–69 years old age group and the data from this study is thus to be considered as representative of Norwegian women. Implementation of an organized population based screening program that includes all aspects of breast cancer, from screening examination, to diagnosis, treatment and follow up is considered to be the main reason for the change in outcome, acknowledging the contributors of advances in technology and training.
References
- Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet 2002; 359: 909–19
- Olsen AH, Njor SH, Vejborg I, Schwartz W, Dalgaard P, Jensen MB, et al. Breast cancer mortality in Copenhagen after introduction of mammography screening: cohort study. BMJ 2005; 330: 220
- The Cancer Registry of Norway. Cancer in Norway, 2005. Oslo: The Cancer Registry of Norway; 2006.
- Vainio H, Bianchini F, IARC Handbook of Cancer Prevention Volume 7 Breast Cancer Screening. Lyon: IARCPress; 2002. http://www.iarc.fr.
- Perry N, Broeders M, deWolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. European Communities, 2006. Printed in Belgium. http://europa.eu.int.
- Hofvind S, Wang H, Thoresen S. Do the results of the process indicators in the Norwegian Breast Cancer Screening Program predict future mortality reduction from breast cancer?. Acta Oncol 2004; 43: 467–73
- Hofvind S, Geller B, Vacek P, Thoresen S, Skaane P. Using the European Guidelines to evaluate the Norwegian Breast Cancer Screening Program. Euro J Epidemiol 2007 ( Jun 27).
- The Ministry of Health and Social Affairs. Regulations on the collection and processing of personal health data in the Cancer Registry of Norway (Cancer Registry Regulations). Oslo: The Ministry of Health and Social Affairs; 2001.
- Tabar L, Tot T, Dean P. Breast Cancer. The art of science of early detection with mammography. Stuttgart, New York: Georg Thieme Verlag; 2005. ISBN 3-13-135371-6 (GTV).
- Hofvind S, Sorum R, Haldorsen T, Langmark F. Incidence of breast cancer before and after implementation of mammography screening. Tidsskr Nor Laegeforen 2006; 126: 2935–8
- Hofvind S, Moller B, Thoresen S, Ursin G. Use of hormone therapy and risk of breast cancer detected at screening and between mammographic screens. Int J Cancer 2006; 118: 3112–7
- Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res Treat 2007.
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: Update of a randomised controlled study. Lancet Oncol 2006; 7: 983–90
- Zahl PH, Strand BH, Maehlen J. Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: Prospective cohort study. BMJ 2004; 328: 921–4
- Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmo mammographic screening trial: Follow-up study. BMJ 2006; 332: 689–92
- Hakama M, Holli K, Isola J, Kallioniemi OP, Karkkainen A, Visakorpi T, et al. Aggressiveness of screen-detected breast cancers. Lancet 1995; 345: 221–4
- Paci E, Miccinesi G, Puliti D, Baldazzi P, De L, V Falcini F, et al. Estimate of overdiagnosis of breast cancer due to mammography after adjustment for lead time. A service screening study in Italy. Breast Cancer Res 2006;8:R68.
- Quiet CA, Ferguson DJ, Weichselbaum RR, Hellman S. Natural history of node-negative breast cancer: A study of 826 patients with long-term follow-up. J Clin Oncol 1995; 13: 1144–51
- Michaelson JS, Satija S, Kopans D, Moore R, Silverstein M, Comegno A, et al. Gauging the impact of breast carcinoma screening in terms of tumor size and death rate. Cancer 2003; 98: 2114–24
- Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993; 328: 1581–6
- Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 2002; 94: 1546–54
- Duffy SW, Tabar L, Vitak B, Day NE, Smith RA, Chen HH, et al. The relative contributions of screen-detected in situ and invasive breast carcinomas in reducing mortality from the disease. Eur J Cancer 2003; 39: 1755–60
- Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA. Breast cancer and atypia among young and middle-aged women: A study of 110 medicolegal autopsies. Br J Cancer 1987; 56: 814–9
- Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 2005; 103: 2481–4
- Zackrisson S, Andersson I, Manjer J, Janzon L. Non-attendance in breast cancer screening is associated with unfavourable socio-economic circumstances and advanced carcinoma. Int J Cancer 2004; 108: 754–60