To the Editors
Adoptive immunotherapy for cancer patients using antigen-specific cytotoxic T lymphocytes (CTL) has been proved safe and has been revealed to induce anti-tumor activity Citation[1], Citation[2]. The principles of adoptive immunotherapy established in animal models have formed the basis for the testing of therapeutic strategies for human tumors Citation[3]. It has been reported that a randomized trial of lymphokine-activated killer (LAK) therapy for progressive or metastatic carcinoma Citation[4–6] resulted in a significant survival advantage. Furthermore, three randomized trials using LAK cells for postoperative supportive therapy of non-small cell lung carcinoma, pancreatic carcinoma, and hepatocellular carcinoma (HCC) were carried out Citation[7–9]. The results confirmed a higher 5-and 9-year survival rate (p < 0.05) and a lower relapse rate in both the pancreatic carcinoma 8 and HCC groups 9 than in their respective control groups. Finally, it was concluded that adoptive cell therapy is a safe and feasible treatment method after surgery for HCC.
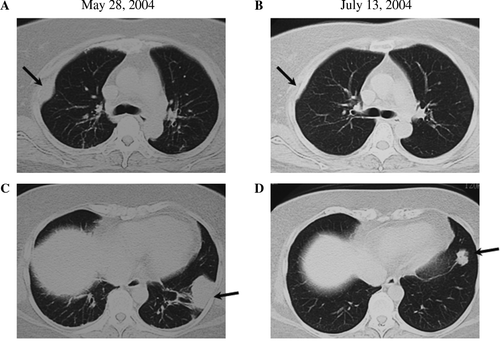
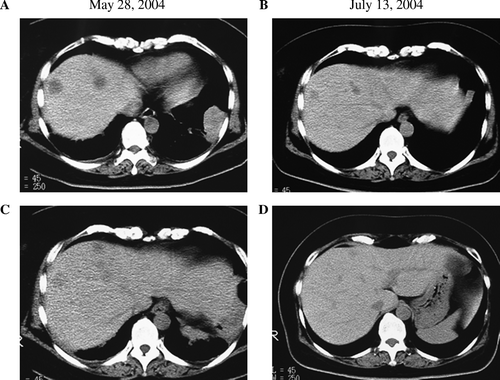
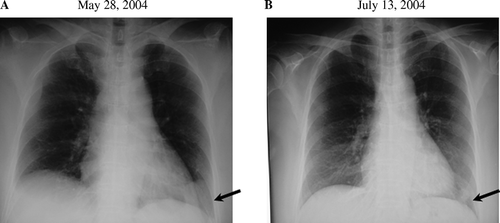
A 56-year-old female patient diagnosed with squamous cell carcinoma of the lung was admitted to our hospital for immunotherapy. A diagnosis of squamous cell carcinoma of the lung had been made 3 years earlier. She had been treated for 2 years with conventional chemotherapy at other hospital, however, which was stopped because of appetite loss and severe general fatigue had developed. For one year before admission, she received no treatment for her lung cancer. She had had several episodes of right chest pain and lumbago in the one year before admission. On admission, a chest x-ray photograph and CT scan showed abnormal shadows in right lower lung field and right pleura (A,2A,C). Further, multiple metastases were found in the bones (lumbar spine and left ribs) (data not shown), right pleura (A), and liver (A,C). A protocol involving chemotherapy and immunotherapy was formulated for her. Nonmyeloablative chemotherapy with cisplatin (10 mg daily, days 1–5) and vincristin (3 mg daily, day 1) was carried out biweekly. Adoptive immunotherapy using autologous cells with anti-tumor activity was conducted weekly. Briefly, peripheral blood mononuclear cells (PBMCs) were collected by gradient sedimentation using Ficoll-Paque and were then primed with an irradiated (30 Gy) squamous cell carcinoma cell line NCI-H2286 from American type culture collection for 5 days. After 5 days of culture with 10 U/ml interleukin-2 (IL-2), the cells were collected and transfused to the patient intravenously. Cytokines have been deemed important in maintaining or augmenting the immune effector function of the lymphocytes of the patient. Between May 28, 2004 and July 18, 2004, the patient was treated with six sequential adoptive cell transfers of autologous lymphocytes reactive to the squamous cell antigen. shows the change in the tumor size in the chest x-ray photograph before (A) and after (B) the therapy. CT scan revealed a tumor shadow in the left lower lobe and a right upper pleural tumor before the therapy (A,C). After the therapy, however, size of the tumor in the left lower lobe and the right pleural tumor shadow decreased significantly (B,D, respectively). Furthermore, this patient had multiple metastatic tumors in the liver (A,C). By treatment with low dose chemotherapy and immunotherapy, not only the primary lung cancer, but also the multiple liver metastases diminished significantly (B,D). During the therapy, no side effects were observed. Adoptive immunotherapy using autologous lymphocytes for the treatment of cancer patients provides the unique advantage of tumor regression with an improvement in the clinical status without severe adverse effects. Several clinical trials have demonstrated the effectiveness of adoptive cell transfer in patients with cancer Citation[4–9]. Finally, our patient received eight sequential adoptive cell transfers accompanied by the nonmyeloablative chemotherapy regimen. A significant reduction in the tumor mass in the lung was observed after the adoptive cell transfer. Moreover, the size of the metastatic tumors in the liver and pleura decreased significantly. Furthermore, with regard to the patient's condition, the use of nonmyeloablative chemotherapy led to minimal toxicity due to chemical agents, and the patient's clinical status never declined.
Figure 1. The change in the tumor size in the chest x-ray photograph before (A) and after (B) the therapy was observed. After the therapy, size of the tumor in the left lower lobe decreased significantly.
References
- Knutson KL, Wagner W, Disis ML. Adoptive T cell therapy for solid cancers. Cancer Immunol Immunother 2005; 54: 721–8
- Dudley ME, Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–75
- Cooley S, June CH, Schoenberger SP, Miller JS. Adoptive therapy with T cells/NK cells. Biol Blood Marrow Transplant 2007; 13: 33–42
- Dillman RO, Soori G, DePriest C, Nayak SK, Beutel LD, Schiltz PM, et al. Treatment of human solid malignancies with autologous activated lymphocytes and cimetidine: A phase II trial of the cancer biotherapy research group. Cancer Biother Radiopharm 2003; 18: 727–33
- Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA 2004; 101: 14639–45
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298: 850–4
- Kimura H, Yamaguchi Y. A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer 1997; 80: 42–9
- Kobari M, Egawa S, Shibuya K, Sunamura M, Saitoh K, Matsuno S. Effect of intraportal adoptive immunotherapy on liver metastases after resection of pancreatic cancer. Br J Surg 2000; 87: 43–8
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000; 356: 802–7