Abstract
The aim of the study is to report the long-term outcome and secondary tumours of early breast cancer patients of adjuvant CNF (cyclophosphamide, mitoxantrone, and 5-fluorouracil) chemotherapy. One hundred and ninety four patients, 185 primary early breast cancer and nine locoregionally recurrent breast cancer patients, were entered onto the trial between May 1986 and November 1993. The therapies included surgery, radiation therapy, adjuvant CNF chemotherapy, and tamoxifen according to hormonal status. Some of patients were treated twice with CMF (methotrexate). The median follow-up time was 12.9 years. Eighty nine (48%) primary breast cancers relapsed, and six locoregional breast cancers relapsed. After 5–10 years the relapse incidence decreased notably. Eighty three patients died of breast cancer, and nine of other causes. Two cases of leukemia, six cases of skin cancer, two cases of Hodgkin's disease, two cases of meningioma, and two cases of endometrial cancer were observed. This article confirms the feasibility of adjuvant CNF for early breast cancer patients. Questions of possible causability of secondary cancer have yet to be explored.
First phase I investigation of mitoxantrone was described year 1980. Thereafter, mitoxantrone has been extensively studied in phase II and III settings. In the eighties, breast cancer chemotherapy included two to four drugs out of cyclophosphamide, methotrexate (M), 5-fluorouracil, adriamycin (A), epirubicin (E), mitomycin C, and mitoxantrone. The combination CNF has been proven to be effective in metastatic breast cancer Citation[1], Citation[2]. Adjuvant chemotherapy and hormonal therapy can prolong survival among patients with early breast cancer Citation[3]. Mitoxantrone has a favourable side effect profile, and we found the CNF combination suitable for adjuvant breast cancer Citation[4]. However, adjuvant chemotherapy can increase secondary tumours Citation[3].
On the basis of the above, we have undertaken a study of 194 breast cancer patients, who received adjuvant CNF/CMF therapy. This article reported the 10-year results. In addition, we studied the role of secondary tumours of mitoxantrone.
Patients and methods
Patient data
Between May 1986 and November 1993, 194 patients were recruited to the study in Kuopio University Hospital, Eastern Finland. Adjuvant chemotherapy was selected at the doctor's discretion. Patients with early breast cancer, but no history of other cancer apart from non-melanoma skin cancer or in situ cancer, were eligible for this trial. Baseline patient characteristics are listed in . The median age was 46 years at the commencement of chemotherapy. Modified radical mastectomy was done in 174 cases, 20 patients had undergone breast-conserving surgery, and axillary dissection was done. Nine patients were treated for locoregionally recurrent breast cancer. The staging was based on UICC criteria Citation[5], stages of primary tumours ranged from 1 (T1cN0M0) to 3B (T4N2M0). Postoperative radiation therapy was administered to 191 patients. The chest wall and regional lymph nodes received a dose of 50 Gy in 25 fractions. Tamoxifen was prescribed for 2 to 5 years in hormone receptor-positive patients. Hormone therapy was often started during the radiation therapy.
Table I. Characteristics of the patients.
Patients received intravenously cyclophosphamide 500 mg/m2, mitoxantrone 10 mg/m2, and 5-fluoro-uracil 500 mg/m2 every 4 weeks. Since February 1990, we replaced the first two cycles with a CMF regimen (methotrexate 40 mg/m2), in order to shorten duration of therapy: patients were treated with concomitant radiation therapy and CMF.
The patients were actively followed-up for 5 to 10 years in the out-patient department of oncology, and thereafter by their own responsible general practitioner. Information on new primary tumours was obtained from the Finnish Cancer Registry, which was founded 1952, and the completeness of registration of new cancer cases has been estimated to be over 98% Citation[6].
Statistical methods
Disease-free survival (DFS) was defined as the interval from the onset of chemotherapy to evidence of relapse or death without relapse. Overall survival (OS) was defined as the time from the onset of chemotherapy to death. Data on patients still alive or disease-free were censored on January 15, 2004. Survival curves were computed according to the Kaplan-Meier method Citation[7]. SPSS for Windows Version 11.5 was used for analysis Citation[8].
Ethics
Approval for the study was obtained from the Finnish Ministry of Social and Health (permit 22/07/2003).
Results
Disease-free survival and overall survival
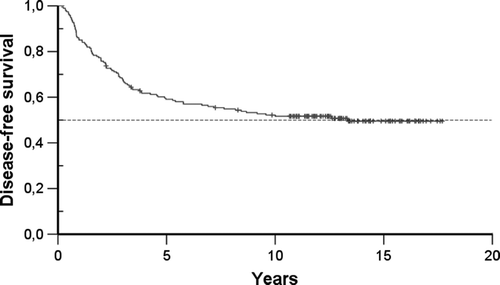
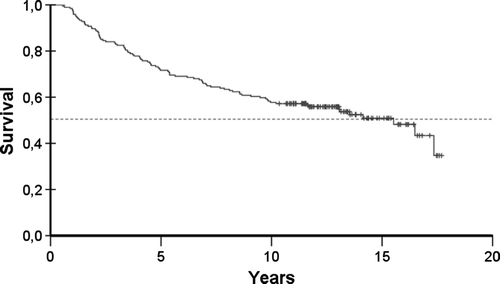
The median follow-up time was 12.9 years (range 10.3–17.7). During the follow-up, 89 (48%) primary breast cancer patients had relapses. Of nine patients with locoregional relapse, six tumours rerelapsed. During the follow-up period, 92 patients (47.4%) died, 83 of breast cancer and nine of other causes. The results translated into 52% and 50% in ten- and 15-year DFS. Ten- and 15-year OS was 57% and 51%, respectively. DFS and OS curves are displayed in and .
Toxicity
All the planned six CNF/CMF cycles were given to 173 (89%) patients, 21 patients stopped treatment prematurely. The patients received mean 5.8 chemotherapy courses. The main reasons of stopping were nausea, leukopenia, and progression of the primary tumour. Four patients stopped chemotherapy for nausea and vomiting. Four patients received leukopenia or aplasia (or even sepsis), but no patient died of cytopenias. Three breast cancer patients presented with relapses during the adjuvant chemotherapy. Single cases included dyspnoea, hepatotoxicity, herpes zoster activation, and diarrhoea.
New cancer events
During the follow-up, 24 patients developed 27 new cancers. Six patients developed a new contralateral breast cancer, two in-situ and four invasive breast cancers, after 1.0 to 11.9 years after the onset of adjuvant chemotherapy. Two leukemias (AML FAB M3 and M4e) were detected after 2.2 and 1.2 years, respectively. One myelofibrosis was diagnosed after 15.4 years. Two patients presented with Hodgkin's disease at 3.2 and 7.2 years, respectively. Six new skin cancers were detected from 2.9 to 14.7 years after the onset of chemotherapy, four cancers on the face and two on the trunk. Five skin cancers were of basal origin, and one case of squamous cell cancer at 11.4 years. Three patients presented with four gynaecological tumours, two ovarian and two endometrial cancers after from 2.1 to 11.6 years. The two endometrial cancer patients used tamoxifen for years. Two meningiomas were detected at 1.7 and 7.5 years, respectively. One rectal and one oesophageal cancer were diagnosed. The squamous cell carcinoma of the opposite main bronchus arose after 14.3 years. The malignant fibrous histiocytoma of the ipsilateral shoulder occurred after 16.6 years. The onset of new cancers is shown in .
Table II. The number and the time of cancers. Asterix (*) means that the patient died of the cancer. Breast cancer deaths are omitted.
Discussion
Results of this retrospective adjuvant CNF study have been reported earlier Citation[4], Citation[9], Citation[10], and this long-term analysis of the trial focused on special issues. Fortunately, breast cancer relapses are rare after 10 years after onset of chemotherapy, although increased breast cancer mortality continues for at least four decades after therapy Citation[11]. Two study groups have compared the efficacy of adjuvant CNF and CMF. Fountzilas et al. Citation[12] randomized 362 patients. After a median follow-up of 51 months, they found no significant advantage in using of mitoxantrone. The Israeli study was smaller with 145 patients. There was no significant difference between the two therapies in terms of OS or DFS Citation[13], Citation[14]. Another study randomized 325 patients with metastatic breast cancer after one chemotherapy had failed, treating with single-agent mitoxantrone or adriamycin. They concluded that mitoxantrone was marginally less active, had less toxicity, but the survival was equal as compared with adriamycin Citation[15]. Our investigators have not recorded cardiac side effects or incidence of cardiac morbidity. No increase in skin toxicity with concurrent CMF and radiotherapy was observed from experience, and the validity of the concurrent chemoradiotherapy was proved with the phase III randomized clinical trial Citation[16].
There is a need for further discussion of secondary cancers in a patient population who received multimodality adjuvant therapy. The more patients relapse of breast cancer, the less patients are at risk of secondary cancer.
Adjuvant chemotherapy for breast cancer is a well-established treatment of proven effectiveness. According to the earlier overview 1998, adjuvant polychemotherapy non-significantly increased non-breast-cancer deaths Citation[17]. The latest systemic overview compared anthracycline-based chemotherapy with CMF. Two regimens did not suggest any substantial difference in efficacy. However, anthracycline-based chemotherapy increased heart disease and haemopoietic neoplasm incidence compared CMF or no chemotherapy, but the difference of events are small, even by chance Citation[3]. We calculated a 1.6% crude incidence of treatment-related MDS/AML in this study. Saso et al. Citation[18] have shown that AML developed from 1.3 to 4.2 years after the onset of chemotherapy, but MDS was found later, from 2.4 to 7 years respectively. These results are in line with other authors. In the present study, six new skin cancers were found, which has not earlier been reported after adjuvant chemotherapy of breast cancer, because benign skin tumours are not perhaps counted. Coutinho et al. Citation[19] and other authors reported that squamous cell carcinomas, basal cell carcinomas of the skin and non-Hodgkin lymphomas are significantly increased after renal replacement therapy. Detecting two meningiomas after adjuvant CNF is surprising. Knuckey et al. Citation[20] reported eight cancer patients with secondary intracranial meningiomas and one with a thoracic meningioma. According to the review, median intervening time was 6 years, and breast carcinoma was diagnosed first in 85% of patients. Physicians should be aware that a solitary brain tumour in a breast cancer patient could be a new primary. One lung cancer and one sarcoma were detected after 14.3 and 16.6 years in the previously irradiated field. The two tumours were considered to be secondary to radiation.
In conclusion, after a 13-year follow-up, our study demonstrated the feasibility of adjuvant CNF chemotherapy for early breast cancer patients. Certain malignancies were increased in these patients. Skin cancer and meningioma caused no deaths, however. The risk for malignancies merits additional investigations because of new promising combination therapies.
Acknowledgements
This study was financially supported by the Finnish Breast Cancer Group.
References
- Alonso MC, Tabernero JM, Ojeda B, Llanos M, Solà C, Climent MA, et al. A phase III randomized trial of cyclo-phosph-amide, mitoxantrone, and 5-fluoro-uracil (CNF) versus cyclophosphamide, adriamycin, and 5-fluorouracil (CAF) in patients with metastatic breast cancer. Breast Cancer Res Treat 1995; 34: 15–24
- Bennett JM, Muss HB, Doroshow JH, Wolff S, Krementz ET, Cartwright K, et al. A randomized multicenter trial comparing mitoxantrone, cyclophosphamide, and fluorouracil with doxorubicin, cyclophosphamide, and fluorouracil in the therapy of metastatic breast carcinoma. J Clin Oncol 1988; 6: 1611–20
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Hirvikoski PP, Kumpulainen EJ, Johansson RT. CNF combination as adjuvant treatment in breast cancer patients is well tolerated. Anti-Cancer Drugs 1997; 8: 376–8
- Sobin LH, Wittekind CH. TNM Classification of Malignant Tumors. New York: Wiley-Liss; 1997. p 123–30.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Acta Oncol 1994; 33: 365–9
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- SPSS for Windows, Release 11.5 (SPSS Inc, ChicagoIL, USA)
- Hirvikoski PP, Kumpulainen EJ, Johansson RT. Hepatic toxicity caused by adjuvant CMF/CNF in breast cancer patients and reversal by tamoxifen. Breast Cancer Res Treat 1997; 44: 269–74
- Kumpulainen EJ, Hirvikoski PP, Pukkala E, Johansson RT. Cancer risk after adjuvant chemo- or chemohormonal therapy of breast cancer. Anti-Cancer Drugs 1998; 9: 131–4
- Brenner H, Hakulinen T. Are patients diagnosed with breast cancer before age 50 years ever cured?. J Clin Oncol 2004; 22: 432–8
- Fountzilas G, Polichronis A, Katsohis K, Gennatas K, Toussis D, Skarlos D, et al. Cyclophosphamide, mitoxantrone, fluorouracil versus conventional CMF as adjuvant treatment in node-positive breast cancer patients. A Hellenic Cooperative Oncology Group Study. Oncology 1996; 53: 137–46
- Ron IG, Wigler N, Borovik R, Brufman G, Rizel S, Shani A, et al. CMF (cyclophosphamide, methotrexate, 5-fluorouracil) versus CNF (cyclophosphamide, mitoxantrone, 5-fluorouracil) as adjuvant chemotherapy for stage II lymph-node positive breast cancer. A phase III randomized multicenter study. Am J Clin Oncol 2001; 24: 323–7
- Ron IG, Wigler N, Borovik R, Peretz T, Shani A, Brenner J, et al. CMF (cyclophosphamide, methotrexate, 5-fluorouracil) versus CNF (cyclophosphamide, mitoxantrone, 5-fluorouracil) as adjuvant chemotherapy for stage II lymph-node positive breast cancer: results by demographic and clinical subgroups. Am J Clin Oncol 2002; 25: 520–2
- Henderson IC, Allegra JC, Woodcock T, Wolff S, Bryan S, Cartwright K, et al. Randomized clinical trial comparing mitoxantrone with doxorubicin in previously treated patients with metastatic breast cancer. J Clin Oncol 1989; 7: 560–71
- Arcangeli G, Pinnarò P, Rambone R, Giannarelli D, Benassi M. A phase III randomized study on the sequencing of radiotherapy and chemotherapy in the conservative management of early-stage breast cancer. Int J Radiat Oncol Biol Phys 2006; 64: 161–7
- Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 1998;352:930–42.
- Saso R, Kulkarni S, Mitchell P, Treleaven J, Swansbury GJ, Mehta J, et al. Secondary myelodysplastic syndrome/acute myeloid leukaemia following mitoxantrone-based therapy for breast carcinoma. Br J Cancer 2000; 83: 91–4
- Coutinho HM, Groothoff JW, Offringa M, Gruppen MP, Heymans HS. De novo malignancy after paediatric renal replacement therapy. Arch Dis Child 2001; 85: 478–83
- Knuckey NW, Stoll J, Jr, Epstein MH. Intracranial and spinal meningiomas in patients with breast carcinoma: Case reports. Neurosurgery 1989; 25: 112–6