Abstract
Low-dose continuous or metronomic chemotherapy with several agents can exert significant antiangiogenic effects, as shown in preclinical studies. Therapy of this kind is generally well tolerated compared with conventional chemotherapy with high, temporally spaced out bolus doses. A critical point emerges when the effects on angiogenesis of low-toxic metronomic doses of chemotherapeutics in preclinical studies are to be transferred to clinical protocols, as there is a risk that a virtually non-toxic dose might also be ineffective; clearly, dose-effect data are important. We therefore sought to investigate whether a dose-dependent response exists in metronomic vinblastine chemotherapy. The surrogate tumor-free rat mesentery model, allowing the study of antiangiogenic effects per se, was used. Following systemically administered metronomic chemotherapy, it closely reflects the indirectly assessed antiangiogenic and growth-retarding effects in a syngenic cancer model. VEGF-A, which is a central proangiogenic factor in most tumors, was administered i.p. to induce angiogenesis in the mesenteric test tissue and, using morphometry, the angiogenesis-modulating effects of vinblastine were assessed in terms of objective quantitative variables. We report that continuous vinblastine treatment with an apparently non-toxic dose (1.0 mg/kg/week or 0.143 mg/kg/day) for 10 days, and a dose that substantially inhibited the physiologic body-weight gain (2.0 mg/kg/week or 0.286 mg/kg/day) for 6 days, demonstrates a dose-response relationship; the high dose significantly suppresses angiogenesis. To our knowledge, no previous study has reported on a dose-dependent antiangiogenic effect by continuous or metronomic vinblastine treatment in a mammalian in vivo model.
A note on terminology
While Browder et al. Citation[1] introduced the term “antiangiogenic chemotherapy”, because of the effect observed, Hanahan et al. Citation[2] shortly thereafter introduced the term “metronomic chemotherapy”, alluding to the frequent dosing schedule used, suggesting effects such as killing tumor microvessel endothelial cells, cancer cells and other cellular constituents of a tumor. In the literature, the term “metronomic” has become almost synonymous with antiangiogenic chemotherapy, apparently implying that the use of low doses generally causes an antiangiogenic effect. Based on recent new findings Citation[3], we suggest that metronomic chemotherapy stands for frequently administered dosing (continuous infusion being viewed as the extreme of metronomic dosing Citation[2]), without any preconceived notion of whether the treatment will suppress or stimulate angiogenesis or produce no effect (the term is used with this meaning in the present paper).
Introduction
Tumors are angiogenesis dependent Citation[4], Citation[5]. Low-dose continuous or frequent antiangiogenic Citation[1], or metronomic Citation[2], chemotherapy, can exert marked angiogenesis-suppressive effects, as well as superior antitumor effects, with no significant toxic side-effects or less severe toxic side-effects, as compared with conventional maximum tolerated dose (MTD) scheduling of chemotherapy Citation[1], Citation[6], Citation[7]. In MTD chemotherapy, the prolonged interval between treatment courses provides an opportunity for new endothelial cells to be recruited, either from existing nearby vasculature or from circulating progenitor endothelial cells, thereby allowing angiogenesis to proceed Citation[8]. The concept of antiangiogenic chemotherapy implies treatment with drug administrations that must be as frequent as possible, i.e. metronomic treatment Citation[1], Citation[2], Citation[7], Citation[9].
Vinblastine, initially isolated from the periwinkle plant Catharanthus roseus, belongs to the vinca alkaloid group of chemotherapeutics that bind to tubulin, the main protein of the cytoskeleton microtubules. This binding prevents tubulin polymerization and the formation of the mitotic spindle leading to cytotoxicity. Earlier preclinical data suggest that the antitumor effect in solid tumors produced by vinca alkaloids could be due in part to a vascular effect Citation[10]. Indeed, vinblastine has proven to have vascular targeting properties, as high-dose vinblastine bolus doses produce a profound and chronic reduction in tumor blood flow in a murine model Citation[11]. An antiangiogenic effect of vinblastine was then plainly shown by Vacca et al. Citation[12], who studied multiple events in human umbilical endothelial cells (HUVECs) related to angiogenesis in vitro. They also reported dose-response antiangiogenic activity by vinblastine in the in vivo embryonic chick chorioallantoic membrane (CAM) assay. Subsequently, numerous reports have demonstrated marked tumor regression in various mouse models following low doses of vinblastine in combination with VEGF-A-targeting drugs Citation[6], Citation[7], Citation[13], Citation[14]. The concept of the antiangiogenic scheduling of chemotherapeutics undoubtedly holds great promise and numerous studies are presently under way using the metronomic concept Citation[14–17].
The great difficulty involved in quantitatively studying the antiangiogenic effect per se of any drug in tumors is, however, a serious obstacle when assessing the effects of putative antiangiogenic agents or treatments Citation[18–20]. Endothelial cells within tumor and non-tumor microvessels are generally considered to be genomically similar and to share signaling pathways Citation[21], even though it has recently been reported that a minority of tumor-associated endothelial cells may be aneuploid in large human tumor xenografts in nude mice Citation[22]. It is furthermore important to remember that tumor vasculature and tumor vessels, preferably in central parts of the tumor, often display chaotic perfusion compared with non-tumor blood vessels Citation[23]. We have been using a non-tumor tissue model that enables the quantitative analysis of angiogenesis-modulating effects following chemotherapy.
The currently used rat mesentery assay displays a number of important relevant features Citation[24]. (a) It is mammalian and adult animals are used. The test tissue is normally vascularized (although sparsely), as are almost all adult normal tissues. (b) No significant physiologic angiogenesis is observed in the test tissue, which is also typical for almost all adult normal tissues. (c) Because the test tissue is extremely thin, it can be analyzed intact under the microscope, thereby allowing detailed recording of the virtually two-dimensional microvasculature in situ. (d) The assay resembles a clinical chemotherapy treatment situation, as it measures the effects of the systemically (p.o., s.c. or i.v.) administered parent cytotoxic molecule and its metabolites, including any modifications that arise from interactions with blood components, vascular endothelial cells and circulating intravascular cells, including platelets that are able to scavenge and store and also selectively release most of the endogenous angiogenesis-regulating proteins, thereby playing an important role in angiogenesis Citation[25]. (e) The test tissue is visceral and should therefore be appropriate for tumor angiogenesis studies, since tumors are prevalent in visceral organs. (f) Angiogenesis in the mesentery test tissue is of the sprouting type, which is predominant not only in mammalian non-tumor angiogenesis but also in tumor angiogenesis. (g) The angiogenic test agent injected i.p. (VEGF-A, for example) comes in direct contact with the test tissue, which is covered on both sides by a layer of flat mesothelial cells, displaying high permeability to compounds within a wide range of molecular weights. (h) The test tissue is exposed to minimum trauma, if any, thereby reducing the risk of inflammation-induced angiogenesis taking place and also excluding wound-healing-induced angiogenesis. (i) The model allows the true quantification and robust statistical analysis of several relevant objective angiogenesis variables Citation[24], Citation[26–28], but it is naturally unable to comprise certain aspects of tumor angiogenesis, as discussed elsewhere Citation[20]. It is also important to consider the fact that multiple proangiogenic factors can be expected to operate in advanced cancers Citation[25]. The present assay of VEGF-A-mediated angiogenesis has, however, been shown closely to reflect the indirectly assessed antiangiogenic and actual growth-retarding effect of metronomic chemotherapy in a syngeneic rat prostate cancer model Citation[29].
Previous studies using this model have revealed that chemotherapeutics administered i.v. as a bolus dose cause distinctly drug-specific, approximately linearly dose-related effects in terms of microvessel spatial extension, density, pattern formation and sprouting Citation[20], Citation[30]. However, in the extreme of metronomic chemotherapy scheduling (i.e. using a continuous infusion schedule), a dose-response relationship is sometimes not present, as recently reported in the case of doxorubicin Citation[3]. Information of this kind is fundamental when designing optimal treatment schedules that are subsequently going to be transferred to clinical trials. Although vinblastine has often been used in preclinical antiangiogenesis models Citation[6], Citation[7], Citation[13], the question of whether or not its antiangiogenic effects are dose related appears not to have been accurately investigated in mammals, which, in contrast to the embryonic avian CAM assay, usually feature sprouting angiogenesis and always incorporate the influence of drug metabolism. We report here that continuously infused vinblastine suppresses VEGF-A-induced angiogenesis dose dependently in the adult rat, but, at an approximately non-toxic dose, angiogenesis was not significantly suppressed in statistical terms.
Material and methods
Animals
Adult male outbred Sprague-Dawley rats (B & K Universal, Sollentuna, Sweden) were acclimatized to a standardized environment for a minimum of 7 days, fed ad libitum and randomly allocated to weight-matched groups with two animals per cage Citation[20]. At the start of the experiments, the mean body weight of the different treatment groups varied insignificantly (between 217.1 g and 219.0 g). Body weight was monitored every 2nd or 3rd day. During a 14-day period, the weight of the vehicle controls increased by a mean of 123.4 g. Chemotherapy-related retardation of the physiologic body-weight gain was regarded as a surrogate evaluation of toxicity, which also included systemic well-being, anorexia and failure to thrive (). The Animal Ethics Committee in Göteborg approved the study. The ethical guidelines that were followed meet the standards required by the UKCCCR guidelines Citation[31].
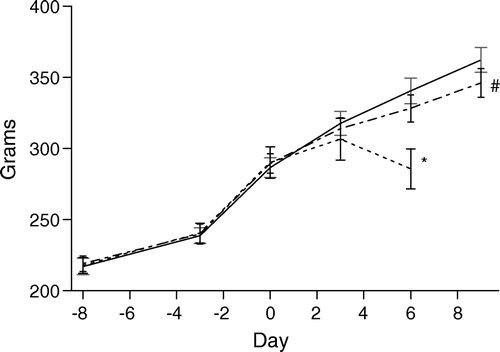
Figure 1. Physiologic body-weight gain in the rats receiving s.c. continuous infusion via an osmotic mini-pump of NaCl-saline vehicle (solid line) for 10 days, vinblastine 1.0 mg/kg/week (dotted line #) for 10 days and vinblastine 2.0 mg/kg/week (dotted line *) for 6 days. All animals had previously received i.p. angiogenic treatment with VEGF-A on days −8 to −3. Vinblastine treatment started on day 0. Mean±SEM.
Angiogenesis treatment
Recombinant rat VEGF164 (564-RV/CF; R&D Systems, Ltd., Oxon, UK), the predominant angiogenic isoform of VEGF-A in rats, was diluted to 96 pmole/ml, frozen and thawed and a volume of 5 ml was injected i.p. Citation[32]. VEGF-A treatment at this dose was given twice daily for 4½ days, i.e. from Monday morning (Day 0) to Friday morning (Day 4). As a result, the VEGF-A very rapidly reached the mesenteric test tissue and its microvascular endothelial cells. This treatment causes a continuous increase in microvessel network proliferation, peaking around Day 21, in the test tissue Citation[32]. It was within this time frame of microvessel network expansion that chemotherapy was given.
Chemotherapy
Initial dose-finding experiments
In order to find non-toxic or almost non-toxic doses that only marginally affected the physiologic body-weight gain in the animals, we performed a dose-finding experiment. Four animals per group were continuously infused s.c. using an osmotic mini-pump (see below) with vinblastine at 0.33, 1.0 and 3.0 mg/kg/week (0.047, 0.143 and 0.429 mg/kg/day respectively); no VEGF-A was given. The chosen dose range was partly based on the use of 0.33 mg/kg/day (2.31 mg/kg/week) as “low-dose metronomic” treatment in mice by Klement et al. Citation[7]. It has been claimed that dose per body surface area (mg/m2) may help in comparing toxicity between species (i.e. laboratory animals with man). For a rat weighing 250 g, the dose in mg/kg times 7 yields an approximate dose in mg/m2 Citation[33]. The doses given by us then correspond to 2.31, 7.00 and 21.0 mg/m2 a week in man. Even if the most commonly used base for the allometric scaling of cytotoxic drug doses between species is “dose per body surface area”, there are clearly instances in which “dose per kg weight” gives a better approximation Citation[34], Citation[35]. In part, this reflects significant species differences with regard to pharmacokinetics and pharmacodynamics, which is clearly the case with vinblastine Citation[36], Citation[37], making the transfer of doses from rats to humans generally problematic. The intention was to use doses that would at most reduce the weight gain so that the body weight at sacrifice was reduced by ≤15% compared with the rapidly growing vehicle control animals.
Chemotherapeutic and vehicle control
The commercially available formulation of vinblastine sulfate (VELBE®) from STADApharm AB, Malmoe, Sweden, was used and diluted in physiologic sodium chloride, which was also used for the vehicle controls.
On Day 7 after the start of the i.p. angiogenic treatment, Alzet® osmotic mini-pumps (Models 2001 or 2ML1; Alzet® Osmotic Pumps, Mountain View, CA, USA) were completely filled under sterile conditions with vinblastine solution. Pumps for controls were filled with the vehicle. One day later, on Day 8, after being stored in sterile 0.9% NaCl (w/v) saline overnight at 37°C, the pumps were surgically implanted s.c. on the back of rats that had been anesthetized with inhaled isoflurane (Forene®, Abbott). The skin incision was immediately sutured after pump implantation. The animals were sacrificed when their body weight following chemotherapy lagged by >15% compared with the rapidly growing controls or, otherwise, on Day 18. No animals were lost.
Angiogenesis quantification
Four membranous (“window”-like) parts of the mesentery from the most distal part of the mesentery, adjacent to the ileocecal valve, were examined after being spread intact on objective slides Citation[32], Citation[38]. Normally, in avascular parts, this tissue measures only ∼5–10 µm in thickness Citation[39], Citation[40] and forms a uniform, almost translucent membrane. The central part of each window is often avascular in untreated animals and the surrounding fatty tissue distinctly delineates each window. The entire vasculature of each intact mesenteric window was visualized immunohistochemically using a primary monoclonal antibody against rat endothelium Citation[3], Citation[41].
Microscopic morphometry and computerized image analysis were employed in a blinded fashion. The following variables were measured objectively in the intact tissue, as described elsewhere Citation[26], Citation[38]: the total area of each mesenteric window was first measured. The vascularized area (VA), a measurement of the spatial extension of the microvasculature, was then assessed as the vascularized area as a percentage of the total window area. The microvascular length (MVL) is a composite measurement of microvessel density in randomly selected view fields within vascularized areas. The total microvascular length (TMVL) was computed as VA times the mean MVL per treatment group. In addition, the following objective variables were measured in randomly selected view fields within the microvessel network: length of individual microvessel segments (Le. MS), i.e. the true distance between two successive branching points. The Le. MS were pooled and ranked in order of size. For statistical comparisons of the treatment groups, the 0–10 and 90–100 percentiles of the Le. MS were used. Other variables that were assessed included the number of microvessel segments (No. MS), i.e. the number of segments per unit tissue area (or tissue volume), the number of microvessel branching points per unit tissue area (No. BP), the index of intersection (In. IS), i.e. the number of microvessel intersections per unit tissue area, and the index of loop formation (In. LF), i.e. the frequency of interconnecting loops per unit tissue area.
Although some of the variables interrelate, it is fair to say that: (i) the VA, MVL and TMVL are primarily measurements of microvessel proliferation; (ii) the No. MS and No. BP reflect both microvessel proliferation and pattern formation; (iii) the Le. MS measures the actual microvessel segment length and (iv) the In. IS and In. LF primarily relate to microvessel pattern formation. For reference, individual mesenteric windows displaying both vascularized and non-vascular parts, as well as the detailed morphology of microvessel segments, intersections, interconnecting loops and sprouts in the virtually 2-dimensional microvasculature, are shown elsewhere Citation[24].
Statistics
The non-parametric Mann-Whitney U-test for unpaired (two-tailed) observations was used. A mean of four windows per animal was used as independent data for each variable except Le. MS in the mesenteric window. For the Le. MS, a percentile (0–10 or 90–100) of all Le. MS in each treatment group (vinblastine 1.0 mg/kg/week, 2.0 mg/kg/week and vehicle) was used, i.e. approximately 150–250 individual Le. MS in each percentile. The criterion for statistical significance was p ≤ 0.05.
Results
Effect of metronomic chemotherapy on physiologic body-weight gain
In the initial dose-finding experiments, the following doses were continuously infused for seven consecutive days and the body weight at sacrifice as a percentage of the vehicle controls is given in parentheses: 0.33 mg/kg/week (99%), 1.0 mg/kg/week (97%) and 3.0 mg/kg/week (85%). For the highest dose, the animals were sacrificed after 6 days’ treatment, due to the fact that their body weight then lagged by 15% compared with the fast growing controls (which was the self-imposed arbitrary limit of body-weight retardation set in this study). In no case did the treatment reduce the body weight in absolute terms. The controls and test animals receiving the two lower doses behaved normally. It was decided to use 1.0 mg/kg/week and 2.0 mg/kg/week in the subsequent angiogenesis experiment.
Dose-related, angiogenesis-modulating effects of metronomic vinblastine chemotherapy
The doses used, 1.0 and 2.0 mg/kg/week, correspond to 0.143 mg/kg/day (1.0 mg/m2/day) and 0.286 mg/kg/day (2.0 mg/m2/day). The chemotherapy did not significantly affect the area of the individual mesenteric windows that were analyzed (data not shown). A direct comparison between control and test groups in terms of the variables given in and can therefore be made, i.e. VA, MVL, TMVL, No. MS, No. BP, In. IS and In. LF ( and and ) and in terms of Le. MS 0–10 percentile. The number of Le. MS analyzed was 2,540 for the vehicle controls, 2,320 for vinblastine at 1.0 mg/kg/week and 1,500 for vinblastine at 2.0 mg/kg/week. For the lower dose (1.0 mg/kg/week), the body weight was hardly affected (96% of control) after 10 days of treatment (), while a statistically insignificant reduction in vascularized area, VA (75% of control), and total microvascular length, TMVL (76% of control), was found. A significant increase in the In. IS to 128% of controls (p ≤ 0.05) and a lengthening of the Le. MS 0–10 percentile cohort (p ≤ 0.0002) was, furthermore, recorded. At the higher dose (2.0 mg/kg/week), a significant relative body weight decrease of 16% compared with the rapidly growing controls was observed, i.e. 1% in excess of what was accepted in our experimental protocol (). Importantly, the reduction in the rate of body-weight gain during the treatment period did not reduce the body weight in absolute terms, as compared with the time of implantation of the s.c. pumps.
Figure 2. Effect of metronomic vinblastine treatment on VEGF-A-mediated angiogenesis in terms of total microvascular length, TMVL (=VA×MVL). Vinblastine or the NaCl-saline vehicle was continuously infused s.c. using an osmotic mini-pump. Note the dose-response relationship, as 2 mg/kg/week strongly suppressed TMVL, while no statistically significant effect was seen after 1 mg/kg/week.
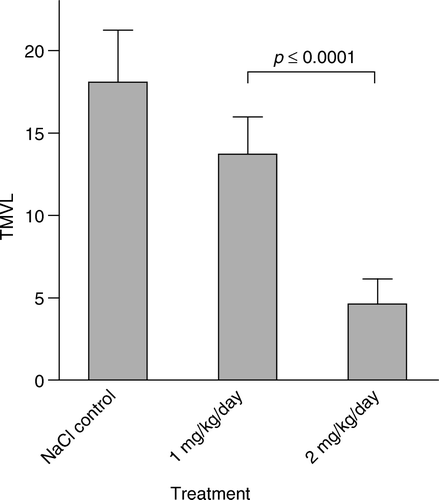
Table I. Effect of metronomic treatment with vinblastine at 1.0 mg/kg/week and 2.0 mg/kg/week (0.143 and 0.286 mg/kg/day) with NaCl saline as the vehicle on VEGF-A-mediated angiogenesis in terms of the vascularized area (VA) and the microvascular length (MVL).
Table II. Effect of metronomic treatment with vinblastine at 1.0 mg/kg/week and 2.0 mg/kg/week (0.143 and 0.286 mg/kg/day) with NaCl saline as the vehicle on VEGF-A-mediated angiogenesis in terms of the number of microvessel segments (No. MS), number of branching points (No. BP), index of intersection (In. IS) and index of loop formation (In. LF).
There was a very strong antiangiogenic effect following treatment with 2.0 mg/kg/week, as the vascularized area, VA, decreased to 43% (p ≤ 0.01), the MVL decreased to 60% (p ≤ 0.005) and the total microvascular length, TMVL, decreased to 25% (p ≤ 0.0001) of controls. Both the No. MS and No. BP were, furthermore, statistically significantly reduced compared with vehicle controls. As shown in , only during the last days before sacrifice did the weight curve for animals given the high dose (2.0 mg/kg/week) bend off markedly from controls, suggesting that the antiangiogenic effect was seen for longer than the last two days.
Compared with controls, the shortest Le. MS, i.e. those of the 0–10 percentile, were significantly lengthened in animals treated with vinblastine at 1.0 mg/kg/week (p ≤ 0.0002) and 2.0 mg/kg/week (p ≤ 0.01). Likewise, the longest Le. MS, those of the 90–100 percentile, were lengthened in animals treated with vinblastine at 1.0 mg/kg/week (p ≤ 0.0001) and 2.0 mg/kg/week (p ≤ 0.01). Following treatment with the low and high dose of vinblastine, the median Le. MS increased to 112% and 105% of control respectively.
Although the effect of the chemotherapy was read on two different days in the same experiment (due to our self-imposed limitation in the experimental protocol with regard to the effect on body-weight gain), the data unambiguously indicate that there was a distinct dose-response effect. In fact, there were significant differences between the 1.0 mg/kg/week dose (0.143 mg/kg/day) following 10 days’ treatment and the 2.0 mg/kg/week dose (0.286 mg/kg/day) following 6 days’ treatment in terms of VA (p ≤ 0.05), MVL (p ≤ 0.01, ), TMVL (p ≤ 0.001, ), No. MS (p ≤ 0.025), No. BP (p ≤ 0.02) and In. IS (p ≤ 0.05, ).
Discussion
We report that continuously administered metronomic vinblastine chemotherapy dose dependently suppresses VEGF-A-mediated angiogenesis in the adult rat. Compared with our previous data on metronomic administration (i.e. continuous infusion) using several chemotherapeutics of various classes in the same experimental setting, the very strong antiangiogenic effects exerted by vinblastine, as observed here, with a statistically significant reduction in both microvessel spatial extension (vascularized area, VA) and density (microvessel length, MVL), have previously only been observed following treatment with paclitaxel and cyclophosphamide, but at doses that affected the physiologic body-weight gain to a lesser degree Citation[3]. It is noteworthy that the present model compares well with other in vivo angiogenesis models in terms of biological, technical and ethical features and that differences in outcome between in vivo angiogenesis models should not be entirely unexpected because of differences in test tissue, species, experimental performance and measuring power Citation[24]. The present results are, however, in line with previous reports from the embryonic in vivo chick chorioallantoic membrane (CAM) assay and also cultured HUVECs Citation[7], Citation[12]. Obviously, any comparison with in vitro data is hampered by the huge difference in experimental complexity. For instance, the influence of drug metabolism and the interaction between various cell types, including platelets Citation[25], that takes place in vivo, cannot be fully captured in vitro. Great care should also be taken when comparing the currently used doses with human doses, as significant differences exist between pharmacokinetic parameters such as half-life and systemic clearance Citation[36], Citation[37], vinblastine also displays non-linear kinetics and there is large intra- and inter-patient variability in humans Citation[36].
Among the vinca alkaloids, differences have been reported in terms of the mechanism of antiangiogenic effects in HUVECs cultured on Matrigel. Vinblastine and vinorelbine appear to exert their effect mainly by cytotoxicity, presumably by preventing tubulin polymerization and disorganization, while it appears that the primary action of vincristine and vindesine is to reduce capillary-like network formation, a mechanism of action that is shared with paclitaxel Citation[42]. Paclitaxel has a similar target of action as the vinca alkaloids, i.e. acting on the microtubules. We have previously reported that metronomic paclitaxel treatment (19 mg/kg/week) for 7 days in rats results in a strong antiangiogenic response, with VA reduced to 44% and MVL to 61% of controls Citation[3]. This is an almost identical antiangiogenic response to that seen in response to 2.0 mg/kg/week of vinblastine presented here. Moreover, in contrast to paclitaxel, vinblastine also exerts a significant effect on the microvessel pattern-related variables No. MS and No. BP ().
Although vinblastine has been used in some of the central preclinical studies of antiangiogenic chemotherapy, comparatively few clinical trials have incorporated vinblastine in their metronomic schedules. To date, most clinical studies of metronomic chemotherapy appear to be based on a fixed low dose of peroral cyclophosphamide in combination with other low-toxicity drugs that may interfere with the angiogenesis process Citation[14]. In a recent phase II study of metronomic chemotherapy, 30% of the patients presenting a variety of advanced malignant diseases demonstrated clinical benefits with weekly vinblastine doses combined with low-dose daily peroral cyclophosphamide and rofecoxib (a selective COX-2 inhibitor) Citation[43].
The very strong antiangiogenic effect seen with the dose of 2.0 mg/kg/week (0.286 mg/kg/day) in the present study was accompanied by a decrease in the rate of physiologic weight gain, the measure used for impact on toxicity. In adult mice, low-dose metronomic (LDM) cyclophosphamide is shown to offer safety advantages compared with conventional maximum tolerated dose (MTD) chemotherapy, in terms of hematological and intestinal toxicity Citation[44]. However, also in these studies, the LDM therapy reduced the rate of physiologic body-weight gain compared with the saline-treated control animals. It thus appears that the gauging of toxicity in adult small rodents by recording decreases in the rate of physiologic body-weight gain is a basically sound method. There are apparently drug- and strain-specific differences among adult mice in terms of the effect on body-weight gain compared with the effects on hematological variables and bone marrow proliferation Citation[45]. It should be noted that adult rats grow considerably more rapidly than adult mice Citation[46]. The effect of chemotherapy on body-weight gain should therefore be a far more sensitive indicator of general toxic effects in rats than in mice and other mammals that do not display significant physiologic growth in adulthood. The way a reduced rate of physiologic body-weight gain in adult rats and mice relates to toxic effects in humans is not yet fully known, however. In the present study, the low vinblastine dose was virtually non-toxic, according to body-weight gain, and insignificantly antiangiogenic in statistical terms.
It is interesting to note that the vinblastine sulfate in the commercially available formula used here was dissolved in sterile water and then further diluted in physiologic NaCl solution to the desired concentration. In a recent study, we made observations suggesting that radical oxygen species (ROS) following chemotherapy may occasionally stimulate angiogenesis in vivo Citation[3]. The question is whether an even more pronounced antiangiogenic effect could have been achieved with vinblastine by including a ROS scavenger in the formulation of the vehicle, thereby reducing the effect of the suggested proangiogenic drive from chemotherapy-induced ROS.
To summarize, this communication reports a dose-response relationship in VEGF-A-mediated angiogenesis in the adult rat following continuous vinblastine chemotherapy. To our knowledge, dose-related effects produced by the continuous infusion or any other type of metronomic scheduling of vinblastine on angiogenesis have not previously been reported in a mammalian in vivo model. We believe that these data should be pertinent when designing schedules for antiangiogenic chemotherapy using vinblastine.
Abbreviations
HUVEC human umbilical vein endothelial cell
i.p. intraperitoneal
i.v. intravenous
MTD maximum tolerated dose
MVL microvascular length, a measurement of microvessel density
p.o. per oral
ROS reactive oxygen species
s.c. subcutaneous
TMVL total microvascular length
VA vascularized area, a measurement of microvessel spatial extension
VEGF-A vascular endothelial growth factor-A
Acknowledgements
The Swedish Cancer Foundation supported this study, grants 02 0503 and 06 0007, together with the King Gustav V Jubilee Clinic Cancer Research Foundation. There are no conflicts of interests to report.
References
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000; 60: 1878–86
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000; 105: 1045–7
- Albertsson P, Lennernas B, Norrby K. On metronomic chemotherapy: Modulation of angiogenesis mediated by VEGF-A. Acta Oncol 2006; 45: 144–55
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31
- Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, et al. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res 2002; 8: 221–32
- Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000; 105: R15–24
- Gately S, Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J 2001; 7: 427–36
- Pasquier E, Honore S, Braguer D. Microtubule-targeting agents in angiogenesis: Where do we stand?. Drug Resist Update 2006; 9: 74–86
- Baguley BC, Holdaway KM, Thomsen LL, Zhuang L, Zwi LJ. Inhibition of growth of colon 38 adenocarcinoma by vinblastine and colchicine: Evidence for a vascular mechanism. Eur J Cancer 1991; 27: 482–7
- Hill SA, Lonergan SJ, Denekamp J, Chaplin DJ. Vinca alkaloids: Anti-vascular effects in a murine tumour. Eur J Cancer 1993; 29A: 1320–4
- Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, et al. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 1999; 94: 4143–55
- Bergers G, Hanahan D. Combining antiangiogenic agents with metronomic chemotherapy enhances efficacy against late-stage pancreatic islet carcinomas in mice. Cold Spring Harb Symp Quant Biol 2002; 67: 293–300
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 2004; 4: 423–36
- Kerbel RS. Antiangiogenic therapy: A universal chemosensitization strategy for cancer?. Science 2006; 312(5777)1171–5
- Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 2006; 108: 452–9
- Stempak D, Seely D, Baruchel S. Metronomic dosing of chemotherapy: Applications in pediatric oncology. Cancer Invest 2006; 24: 432–43
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: Microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 2002; 94: 883–93
- Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: From the research laboratory into the oncology clinic. Ann Oncol 2002; 13: 12–5
- Lennernas B, Albertsson P, Lennernas H, Norrby K. Chemotherapy and antiangiogenesis–drug-specific, dose-related effects. Acta Oncol 2003; 42: 294–303
- StCroix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science 2000; 289(5482)1197–202
- Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res 2004; 64: 8249–55
- Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer 2002; 2: 266–76
- Norrby K. In vivo models of angiogenesis. J Cell Mol Med 2006; 10: 588–612
- Folkman J. Angiogenesis: An organizing principle for drug discovery?. Nat Rev Drug Discov 2007; 6: 273–86
- Norrby K. Microvascular density in terms of number and length of microvessel segments per unit tissue volume in mammalian angiogenesis. Microvasc Res 1998; 55: 43–53
- Norrby K. Angiogenesis: New aspects relating to its initiation and control. Apmis 1997; 105: 417–37
- Naslund I, Norrby K. NO and de novo mammalian angiogenesis: Further evidence that NO inhibits bFGF-induced angiogenesis while not influencing VEGF165-induced angiogenesis. Apmis 2000; 108: 29–37
- Lennernas B, Albertsson P, Damber JE, Norrby K. Antiangiogenic effect of metronomic paclitaxel treatment in prostate cancer and non-tumor tissue in the same animals: A quantitative study. Apmis 2004; 112: 201–9
- Albertsson P, Lennernas B, Norrby K. Chemotherapy and antiangiogenesis: Drug-specific effects on microvessel sprouting. Apmis 2003; 111: 995–1003
- United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia. 2nd ed. Br J Cancer 1998;77:1–10.
- Norrby K. Vascular endothelial growth factor and de novo mammalian angiogenesis. Microvasc Res 1996; 51: 153–63
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 1966; 50: 219–44
- Davidson IW, Parker JC, Beliles RP. Biological basis for extrapolation across mammalian species. Regul Toxicol Pharmacol 1986; 6: 211–37
- Dixon RL. Problems in extrapolating toxicity data for laboratory animals to man. Environ Health Perspect 1976; 13: 43–50
- Rahmani R, Zhou XJ. Pharmacokinetics and metabolism of vinca alkaloids. Cancer Surv 1993; 17: 269–81
- Zhou XJ, Martin M, Placidi M, Cano JP, Rahmani R. In vivo and in vitro pharmacokinetics and metabolism of vincaalkaloids in rat. II. Vinblastine and vincristine. Eur J Drug Metab Pharmacokinet 1990; 15: 323–32
- Norrby K. Basic fibroblast growth factor and de novo mammalian angiogenesis. Microvasc Res 1994; 48: 96–113
- Norrby K, Bergstrom S, Druvefors P. Hyperplasia and growth of the true mesentery in the diabetic rat. Acta Pathol Microbiol Immunol Scand [A] 1983; 91: 195–202
- Norrby K, Franzen L. A tissue model for the study of cell proliferation in vitro. In Vitro 1980; 16: 31–7
- Norrby K, Mattsby-Baltzer I, Innocenti M, Tuneberg S. Orally administered bovine lactoferrin systemically inhibits VEGF(165)-mediated angiogenesis in the rat. Int J Cancer 2001; 91: 236–40
- Hayot C, Farinelle S, De Decker R, Decaestecker C, Darro F, Kiss R, et al. In vitro pharmacological characterizations of the anti-angiogenic and anti-tumor cell migration properties mediated by microtubule-affecting drugs, with special emphasis on the organization of the actin cytoskeleton. Int J Oncol 2002; 21: 417–25
- Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res 2006; 12: 3092–8
- Emmenegger U, Man S, Shaked Y, Francia G, Wong JW, Hicklin DJ, et al. A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res 2004; 64: 3994–4000
- Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 2005; 106: 3058–61
- Poiley SM. Growth tables for 66 strains and stocks of laboratory animals. Lab Anim Sci 1972; 22: 758–79