Abstract
Introduction. Radioiodine and most cytostatic treatments are contraindicated in pregnancy. Still, inadvertent therapy does occur. Radioiodine was given to two pregnant women with Graves’ disease and thyroid cancer respectively, both in their 20th gestational week. Routine pregnancy tests based on urinary β-hCG had failed to indicate pregnancy in both cases. Methods. Estimation of doses to the foetuses and foetal thyroids. Scrutiny of pregnancy testing. Results and Conclusions. Doses to foetal thyroids were ablative (250–600 Gy). Total foetal dose in the Graves’ patient was 100 mGy and compatible with survival, whereas a foetal dose of approximately 700 mGy together with induced hypothyroidism was fatal for the foetus of the cancer patient. Routine pregnancy tests may fail early and late in pregnancy. The possibility of pregnancy should be considered in all fertile women before therapy with radionuclides or cytostatic regimens, and a clinical investigation undertaken on wide indications with determination of serum β-hCG, preferably together with an ultrasound examination.
In 1998, we reported a case where a woman had been treated with radioiodine for Graves’ disease during pregnancy Citation[1]. Since then, only single reports regarding foetal uptake of radionuclide after radioiodine treatment of a pregnant woman have been published Citation[2]. There thus remain many unanswered questions, regarding the radiation dose to the foetus and the clinical outcome in this unfortunate clinical situation.
Now, 18 years after the first incident, we have experience from a second case where a woman received radioiodine during an undiagnosed pregnancy. We therefore feel obliged to emphasize the pitfalls of diagnosing a pregnancy early, and after the 18th gestational week, and suggest strict clinical routine measurements in radioiodine treatment of women of fertile age. Furthermore, the two incidents bear not only similarities – the exposure to radioiodine was in the same gestational week – but also important dissimilarities; in the second case, the mother had undergone thyroidectomy and was hypothyroid when radioiodine was given, and the amount of administered radioactivity was higher. We therefore feel it is important to report on this second case and compare it with the previous one.
Case 1
A 43-year-old woman with Graves’ disease was treated with 500 MBq 131I when she was in her 20th gestational week, corresponding to a foetal age of 18 weeks. Her pregnancy was discovered 10 days after the administration of the radiopharmaceutical, and repeated gamma camera examinations of the abdomen were performed, showing the foetal thyroid 10, 11, 12, 13 and 18 days after the administration. These serial measurements made it possible to determine the effective half-life of the radionuclide and hence to estimate the mean absorbed dose to the foetus and foetal organs (the whole foetal body 100 mGy; the foetal thyroid gland 600 Gy). The child, a boy, was born at full term and was healthy except for a total loss of thyroid function. Prior to the event, the woman and her referring gynaecologist were informed about the absolute contraindication of radioiodine treatment in pregnancy. The woman had performed negative pregnancy tests both early and late in her pregnancy Citation[1]. Today, the boy is 18 years old, performing well at school, and aiming at university studies. He has not been diagnosed with cancer or any other serious disease (O Westphal, personal communication).
Case 2
In 2005, a 28-year-old woman received radioiodine treatment for remnant ablation after total thyroidectomy for thyroid cancer. Twenty-five days after the administration, it was discovered that the patient was pregnant. From abdominal ultrasound it was estimated that the radioiodine treatment was given in gestational week 20, that is, at a foetal age of 18 weeks.
The patient already had a 7-year-old child and did not desire a further pregnancy, so her partner had been using barrier protection (condom). Because of missed menstrual bleedings during the five months preceding the radioiodine therapy, she had performed two negative pregnancy tests early after missed periods. In connection with the radioiodine therapy, two more negative pregnancy tests were performed (“PREG” pregnancy test, ACO, Sweden, sensitivity hCG: 50 mlU/ml) under supervision of medical staff.
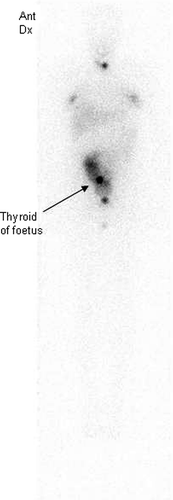
The patient was found to have a multifocal differentiated papillary thyroid cancer, WHO stage pT1N1M0. The choice of radioiodine therapy was based on the finding of metastases in four of lymph nodes together with periglandular growth. An activity of 3 700 MBq 131I was prescribed. The patient suffered from mild asthma but was otherwise healthy. Before radioiodine therapy, thyroid hormone substitution was withdrawn (T3 medication for 4 weeks, and 2 weeks without thyroid hormones) in order to achieve a high endogenous TSH stimulation. On the day of radioiodine therapy, the serum TSH concentration was 78 mIU/L, and the serum thyroglobulin concentration 4.9 µg/L. A scintigraphy performed 6 days after administration showed two areas of low uptake corresponding to the thyroid bed. Minor uptake was also demonstrated in the breasts. In addition, an area with increased uptake in the abdominal region was eventually interpreted as a foetal thyroid ().
Figure 1. Gamma camera examination 6 days after administration of 3 700 MBq 131-I in Case 2. Note small uptake in the thyroid bed, uptake in mammary glands, and uptake in the fetal thyroid and fetal body/amniotic fluid.
When the pregnancy was finally detected, an ultrasound investigation revealed a foetus of normal appearance with an estimated foetal age of 21 weeks and 5 days (gestational week 23). The patient applied to the Swedish National Board of Health to have an abortion, and the pregnancy was medically terminated in the 24th gestational week. The child was dead at delivery. The pregnancy was revealed too late to allow further gamma camera examinations.
Dose calculations
Absorbed dose to the foetal thyroid in Case 1
Quantification of the 131I uptake in the foetal thyroid was made from gamma camera measurements Citation[1]. The first measurement, at day 10, showed an activity of 0.6 MBq, and from subsequent measurements the effective half-life was calculated as 2.5 days, corresponding to a biological half-life of 3.6 days. The initial uptake was calculated as 10 MBq, that is, 2.0% of the administered activity, the cumulative activity being 900 MBq×hr. The thyroid mass was assumed to be 143 mg at a foetal age of 18 weeks Citation[3]. Using an S-value of 0.65 Gy/MBq×hr Citation[4], the absorbed dose to the thyroid was calculated as 600 Gy.
Absorbed dose to the foetal thyroid in Case 2
Quantification of 131I content in the foetus was made from a gamma camera measurement at a single occasion on day 6. The activity in a ROI (region of interest) approximately corresponding to the size of the foetus was 5 MBq, and the activity in a hot spot interpreted as the foetal thyroid was 1 MBq, corresponding to a thyroid uptake of 0.027%. Assuming a biological half-life in the thyroid in the range 6–1d, the cumulative activity range was calculated to be in the range 400–3 000 MBq×hr for 3 700 MBq administered activity. Using an S-value of 0.65 Gy/MBq×hr, the absorbed dose to the thyroid was estimated to be in the range 260–2 000 Gy. Using the same biological half-life as measured in Case 1, that is, 3.6 days, the absorbed dose was calculated as 300 Gy.
Discussion
β-hCG during pregnancy, pregnancy testing
A routine pregnancy test from urine can be used to confirm a suspected pregnancy, but can obviously not be used to completely rule out pregnancy. β-hCG can be detected in the urine of the pregnant woman 8–10 days after ovulation. In the two cases presented in this paper, the first tests were performed early in pregnancy, which might explain the failure to diagnose the woman's pregnancy. Obviously, the 10-day rule should be followed Citation[5].
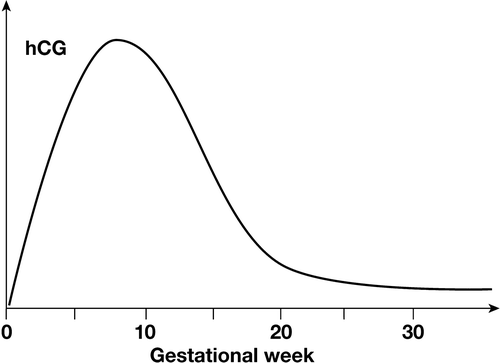
The serum β-hCG level peaks at 8–10 weeks of gestation, reflected in a peak in urinary β-hCG. At the 13th week of gestation, the structure is changed, with a decreased degree of sialinization, and a shorter half-life of the hormone Citation[6]. This decrease in half-life, added to a decrease in production rate, will cause a rapid decrease in the β-hCG levels in both serum and urine (). The β-hCG level can be low from mid term, especially in case of a male foetus Citation[7], and it was early recognized in the evaluation of pregnancy tests that pregnant women may show negative urinary tests from the 18th gestational week. The tests are today more sensitive, but pregnancies from the 18th week may still be missed (L Wide, personal communication). Some tests used today react with both the free β-subunit and with intact β-hCG. This may have an advantage in detecting very early pregnancies, but may negatively affect the sensitivity of the test in detecting a pregnancy after the 18th week, the free β-subunit concentration being most markedly increased in the beginning of the pregnancy.
The possibility of pregnancy should be considered in all fertile women before therapy with radionuclides or cytostatic regimens, and a clinical investigation aimed at excluding a pregnancy undertaken on wide indications. Routinely available pregnancy tests are, however, designed to be used at the very early stage of a pregnancy, and not to be used as a precaution to rule out an (unlikely) pregnancy in midterm. The concentration of β-hCG is approximately the same in serum and urine, but the urinary concentration may be affected by several factors including intake of water. Therefore, in the clinical setting discussed in this paper, a determination of the serum β-hCG is preferred over the urinary β-hCG determination used in routine pregnancy tests, preferably together with an abdominal/vaginal ultrasound examination.
The consequence of radioiodine therapy in pregnancy
The foetal thyroid begins to develop at 5–6 weeks, with follicle and colloid production at 10–12 weeks of gestation. Inadvertent therapy with 131I has been associated with a normal foetus when radioiodine was administered before the 10th week of gestation Citation[8]. Radioiodine administration at a time later than this may cause thyroid ablation, resulting in foetal and neonatal hypothyroidism with severe and irreversible consequences, including mental retardation, if the condition is not immediately recognized. From our first case we found that the consequence was foetal thyroid ablation, while the total foetal absorbed dose of about 100 mGy did not reach the limit for allowing early abortion. In the second case, however, the consequences were far more serious. Besides risks from radiation, it must be emphasized that the foetus was also exposed to risks from anaesthesia early in pregnancy, and to low levels of the thyroid hormones, which are particularly important for the development of the central nervous system Citation[9]. In addition, the hypothyroid state may have affected the initial iodine uptake in the foetal thyroid and foetal body/amniotic fluid.
Dose calculations
An accurate calculation of the absorbed dose to the foetal thyroid can only be performed if the initial iodine uptake, effective half-life, and thyroid mass are known. In the ICRP 88 publication Citation[10], absorbed doses to foetuses of various ages are given in relation to maternal 131I intake. The calculations are based on a biokinetic model with parameter values estimated to be valid for a euthyroid pregnant woman. The model predicts a thyroid uptake of 0.3% and an effective half-life of 5.7 days for an 18-week foetus. These values differ from the values found in our calculations regarding Case 1 (2.0% and 2.5 days, respectively). This discrepancy explains the difference between the absorbed dose coefficient given by ICRP 88, 0.45 Gy/Bq, (interpolated value), and that given by us, 1.2 Gy/MBq. The thyroid mass used in the two calculations is similar, 133 mg in ICRP 88 and 143 mg in our report. An ultrasound study of the thyroid volume of 75 foetuses has indicated a thyroid weight of 80±50 mg at gestational week 20–23 Citation[11], suggesting an underestimation of present absorbed dose values.
In Case 1, all activity was seen in the foetal thyroid and none in the foetal body. In Case 2, a clearly visible diffuse uptake was seen, representing a substantial amount of 131I in the foetal body/amniotic fluid. We assume that also in Case 2 all foetal activity was first taken up by the foetal thyroid and then by fast turnover in the thyroid (i.e. a short half-life) excreted in the amniotic fluid. This may explain why the thyroid uptake at day 6 was lower in Case 2 than in Case 1. A fast turnover in the foetal thyroid can be explained by an increased TSH drive during induced hypothyroidism.
In Case 2 the dose estimation was based on the single uptake measurement on day 6 and the assumption that the effective half-life was in the range 1–6 days, where the shorter half-life is thus the most plausible. Despite uncertainties in the absorbed dose estimation, it can be concluded that the absorbed dose to the foetal thyroid was high, probably more than 250 Gy, and should have been ablative. It is noteworthy that the dose calculated from this case is remarkably higher than the supposed dose from ICRP calculation.
The absorbed dose to the foetal body in Case 1 was estimated at 0.2 mGy/MBq Citation[1], with the major contribution coming from 131I in the foetal thyroid. This dose coefficient applied to Case 2 gives an absorbed dose to the foetal body of 700 mGy. The dose however is very uncertain, due to the lack of biokinetic data. A high absorbed dose together with induced hypothyroidism proved to be lethal for the foetus.
Conclusion
Treatments with radionuclides and most cytostatic treatments are absolutely contraindicated in pregnancy. Still, inadvertent radioiodine therapy in pregnancy does occur. Therefore, the possibility of pregnancy should be considered in all fertile women before therapy with radionuclides or cytostatic regimens, and a clinical investigation aimed at excluding a pregnancy undertaken on wide indications. This issue has currently become even more important, since contemporary treatment of young women (<40 years) with early breast carcinomas includes adjuvant cytostatic regimens. There may be other treatment options to discuss with the mother if a pregnancy is confirmed prior to treatment Citation[12].
Commercial pregnancy tests based on urinary β-hCG are not suitable for use later in pregnancy that is after 18 weeks. Instead, serum β-hCG should be analyzed, preferably in addition to an abdominal/vaginal ultrasound examination.
In the case of radioiodine therapy for hyperthyroidism or thyroid cancer in mid gestation, the dose to the foetal thyroid can be high (20–600 Gy) resulting in ablation of the foetal thyroid. In the case of radioiodine therapy for thyroid cancer, a total foetal dose of approximately 700 mGy together with hypothyroidism is likely to be fatal for the foetus.
Acknowledgements
We would like to express our gratitude to Professor Leif Wide, Department of Medical Sciences, Uppsala University, Uppsala, for valuable information regarding diagnostic procedures in pregnancy, Dr. Otto Westphal, Department of Pediatrics, Sahlgrenska University Hospital, Göteborg for clinical information regarding Case 1, and Dr. Lennart Darte, Department of Radiation Physics, Lund University Hospital, for having shared his great knowledge in “Commission works on Radiological Protection”. We would also like to thank Ms. Karin Wingardh for excellent technical assistance.
References
- Berg GEB, Nyström EH, Jacobsson L, Lindberg S, Lindstedt RG, Mattsson S, et al. Radioiodine treatment of hyperthyroidism in a pregnant woman. J Nucl Med 1998; 39: 357–61
- Evans PMS, Webster J, Evans WD, Bevant JS, Scanlon MF. Radioiodine treatment in unsuspected pregnancy. Clin Endocrinol 1998; 48: 281–3
- Elsasser U, Henrichs K, Kaul A, Reddy AR, Roedler HD. Specific absorbed fractions and S-factors for calculating absorbed dose to embryo and fetus. In: Schlafke-Stelson AT, Watson EE, Proceedings in the fourth international radiopharmaceutical dosimetry symposium. Oak Ridge, TN: Oak Ridge Associated Universities; 1986. p 155–165.
- Watson E. Radiation absorbed dose to the human fetal thyroid. In: Schlafke-Stelson AT, Watson EE, Proceedings of the fifth international radiopharmaceutical dosimetry symposium. Oak Ridge, TN: Oak Ridge Associated Universities; 1991. p 179–187.
- Watson WS. Radioiodine therapy and pregnancy. J Nucl Med 2005; 46: 1408–9
- Sutton JM. Charge variants in serum and urine hCG. Clin Chim Acta 2004; 341: 199–203
- Steier JA, Myking OL, Bergsjo P. Correlation between fetal sex and human chorionic gonadotropin in peripheral maternal blood and amniotic fluid in second and third trimester normal pregnancies. Acta Obstet Gyneol Scand 1999; 78: 367–71
- Stoffer SS, Hamburger JI. Inadvertent 131I therapy for hyperthyroidism in the first trimester of pregnancy. J Nucl Med 1976; 17: 146–9
- Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol 2004; 151: U45–U48
- ICRP 88. Doses to the embryo and fetus from intakes of radionuclides by the mother. Ann ICRP 2001;31:1–3.
- Ho SSY, Metreweli C. Normal fetal thyroid volume. Ultrasound Obstet Gynecol 1998; 11: 118–22
- Cardonic E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncology 2004; 5: 283–91