Abstract
Purpose. To assess the acute toxicity profile of whole pelvis IMRT (WP-IMRT) for localized prostate cancer. Materials. Eighty seven patients treated with definitive WP-IMRT at UTMB from May 2002 to November 2006 were retrospectively reviewed. Treatment consisted of two sequential phases, WP-IMRT to 54 Gy at 1.8 Gy per fraction to the pelvic nodes and seminal vesicles and 60 Gy at 2 Gy to the prostate, and a separate external beam boost, 3DCRT or IMRT, to bring the dose to the prostate to 76 Gy. Acute toxicity was prospectively scored weekly during treatment and at 3 month follow-up according to CTC v2.0 for 10 genitourinary (GU) and gastrointestinal (GI) domains. The proportion of patients experiencing a given level of peak acute toxicity at a given point is reported. Results. Treatment was feasible with delivered doses to PTVs not significantly lower than planned ones and with only two patients experiencing treatment gaps longer than 5 days. About 2/3 and 1/10 of the patients experienced peak grade 2 and grade 3 reactions at least once during RT, respectively. Frequency/urgency (Grade 2+: 37.9%) and diarrhea (36.7%) were the most prevalent symptoms followed by proctitis (21.8%) and dysuria (16.1%). GI reactions were generally shorter lasting compared to GU ones which accumulated progressively during treatment. At 3 months, almost half of the patients were asymptomatic and most of observed reactions (89.2%) were mild, with GI ones more likely to be fully resolved (92.5%) than GU ones (68.7%, χ2, p=0.001). Conclusion. Our approach is dosimetrically and clinically feasible with intense, but transient, acute toxicity.
An attempt to clarify whether it is beneficial to include pelvic lymph nodes in the initial treatment volume for localized prostate cancer has been recently provided by RTOG trial 9413 Citation[1]. Preliminary results showed that, within the context of short course androgen deprivation, patients with a risk of pelvic lymph node (PN) involvement equal to or greater than 15% and who receive PN treatment with standard 4-field box technique (4FBT) have an advantage in progression free survival over those treated on the prostate only (PORT) Citation[1]. Though the advantage of treating PN decreased at a subsequent analysis Citation[2], the authors were able to illustrate a correlation between the size of portal fields (that would correspond to the amount of pelvic nodes treated) and outcome Citation[3], supporting the need for ‘comprehensive’ nodal coverage.
Interestingly, the same paper also shows that extending the 4FBT fields to cover the PN may have a cost in terms of acute toxicity over PORT. In particular, grade 2+ gastrointestinal (GI) toxicity are 46.6% and 20.2% for patients treated to the whole pelvis and prostate only, respectively (p < 0.001) Citation[3]. These data may be particularly interesting in view of the fact that, in several series (summarized by Garg et al) Citation[4] acute GI toxicity has been shown to predict for late GI toxicity.
We, and other groups, have investigated the role of IMRT during the ‘pelvic‘ or initial phase of treatment of localized prostate cancer Citation[5–8]. While it should be noted that IMRT is not needed in every single patient and that standard 4FBT and its variants can be an equally effective option in some patients Citation[9], whole-pelvis IMRT (WP-IMRT) has the unique capability to maximize the therapeutic ratio over 4FBT, achieving the most comprehensive target coverage without negatively impacting the dose to rectum/bladder Citation[5], Citation[9]. However, despite its widespread use Citation[10], acute toxicity rates associated with WP-IMRT have been reported in only a few patients and with conflicting results Citation[11], Citation[12].
At our institution we developed a WP-IMRT strategy for selected patients with localized prostate cancer in May 2002 and here we report the acute toxicity rates obtained in all patients (n = 87) recruited up to November 2006.
Methods
Patients
Whole pelvis IMRT has been routinely offered at UTMB as part of definitive treatment of localized (T1-4N0-xM0) prostate cancer with at least 15% risk of pelvic nodal disease according to the Roach formula Citation[13] since May 2002.
Through November 2006, 98 patients were referred to our department being potentially eligible for WP-IMRT. Of these patients, one received standard four-field box due to inability to hold the supine position for longer than 10 minutes secondary to low back pain, leaving 97 patients treated with WP-IMRT. Of them, ten were further excluded from the present analysis because they were treated within an in-house protocol of either WP-IMRT followed by HDR boost (8 pts) or WP-IMRT combined with a simultaneous integrated boost to the prostate (2 pts). Therefore 87 patients were treated with WP-IMRT and sequential-external beam RT boost to the prostate to 76 Gy, and all of them are included in the present analysis that it has been approved by the Institutional Review Board of the University of Texas Medical Branch.
Simulation, organ contouring and planning
Patient set-up, simulation and organ contouring have been previously reported in detail Citation[5], Citation[9]. Briefly, patients were simulated supine with an alpha cradle immobilizing the lower extremities. Patients were instructed to present for simulation and treatment with an “empty” rectum. The bladder had to be voided ½ − 1 h before simulation and each treatment.
The prostate apex was identified through urethrography, or lately MRI.
A planning CT was obtained at 5-mm slice thickness from the top of the iliac bone to at least 5 cm below the base of the penis; a slice thickness of 3 mm was obtained for the cross-sectional slices containing the prostate and seminal vesicles (SV). Images were transferred to the Pinnacle3 [Philips Medical Systems, Madison, WI] treatment planning system.
Clinical target volumes 1 and 2 (CTV1, CTV2) were defined as the prostate plus 1–1.5 cm of SV and the prostate with entire SV and pelvic nodes, respectively. CTV2 included the obturator and hypogastric, internal and external iliac (from the bifurcation of the common iliac artery at the level of the top of the sacroiliac joints to the point where the external iliac artery crossed the inguinal ligament), the lower part of the common iliac nodes (caudal to L5/S1), and the presciatic and presacral (anterior to the first and second sacral segments) nodes as previously reported Citation[9].
The prescription dose for CTV1 was 76 Gy at 2 Gy per fraction, daily. CTV2 was treated to 54 Gy at a daily dose per fraction of 1.8 Gy.
The rectum was contoured from above the anal verge to the rectosigmoid junction, including its contents as previously defined Citation[14]. The intestinal cavity (IC) was defined and delineated as the space encasing bowel loops starting cranially from the top of iliac crests.
Clinical target volumes were expanded to obtain corresponding planning target volumes (PTVs). CTV2 was always expanded 1 cm in all directions; for CTV1 margin size was correlated with the use of 3 fiducials for daily repositioning: for 61 patients without fiducials, margins were 1.0 cm craniocaudally, 1.3 cm anteriorly and laterally, and 0.8 cm posteriorly at rectum interface; for 50 patients with fiducials, margins were reduced to 1.0 cm in all directions but posteriorly (0.5 cm) (‘tight margins’).
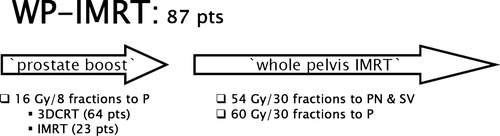
As mentioned above, the planning process for WP-IMRT combines two sequential phases, an initial one to cover the prostate PTV or PTV1 to 16 Gy in 8 fractions and a second one to deliver, in 30 fractions, 60 Gy to PTV1 and 54 Gy to PTV2 ().
The initial boost was tentatively planned with a 6-field conformal approach with gantry angles of 240°, 270°, 300°, 60°, 90° and 120°, using 18 MV photons; a forward planning field-in-field IMRT technique was utilized to create uniformity of dose within the prostate PTV. In 23 patients who were not able to meet dose volume objectives on either OARs or PTVs with 3DCRT and whose plan was considered unacceptable, an IMRT boost with inverse planning was employed as detailed elsewhere Citation[9].
The second or ‘pelvic’ phase was always delivered with inverse planning IMRT with 8 coplanar and non-opposed gantry angles (220°, 260°, 300°, 340°, 20°, 60°, 100° and 140°) fields and 6 MV photons.
The way the two sequential phases were optimized has been reported in detail elsewhere Citation[9].
Dose volume objectives for the rectum and bladder are illustrated in . Regarding the intestinal cavity, we initially included it in the ‘unspecified tissue’ or anti-PTV with a maximum dose constraint of 35 Gy for the portion non-overlapping with the PTV. After a preliminary analysis on the first 24 patients Citation[15], the IC volume was separated from unspecified tissue. Secondary dose volume objectives were placed on the portions overlapping and not overlapping with pelvic node PTV. The former included a uniform dose objective of 55 Gy; the latter, the dose to 50%, 30%, 20% and any portion of IC to not exceed 10 Gy, 25 Gy, 35 Gy and 55 Gy, respectively.
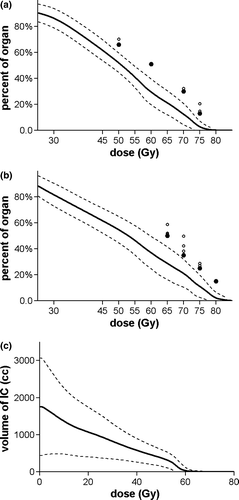
Figure 2. Average (+SD) planned rectal (a), bladder (b) and intestinal cavity (c) DVH for 87 patients treated with WP-IMRT. Solid circles represent planning dose objectives. Open circles correspond to values exceeding dose volume objectives.
We pursued marginal coverage of all PTVs, defined as less than 100% but more than 95% of the volume receiving at least 95% of the dose (i.e., V95 ≥95%)Citation[16].
Dosimetric data on PTV1 and PTV2 coverage as well as selected OARs (bladder, rectum and intestinal cavity) were tentatively extracted for each patient from the original treatment plan, converted into a Microsoft Excel file, and recomputed as cumulative DVH data.
Acute toxicity
Patients were seen weekly during treatment and acute toxicity was prospectively scored at each visit according to the CTC v2.0 scale () by eight observers. Ten items or domains were considered; five gastrointestinal ones: diarrhea, colitis, proctitis, rectal bleeding, proctalgia; five genitourinary (GU) ones: hematuria, dysuria, incontinence, frequency/ urgency and urinary retention. In order to maximize consistency among observers, an acute toxicity form with the complete explanation of each grade as per had to be filled during each examination while interviewing the patient.
Table I. Acute toxicity items selected for the present study and CTC v2.0 scoring.
Pretreatment symptoms were prospectively collected only for two items, incontinence and frequency/urgency. Patients returned for the first follow-up at 3 months after treatment completion. Dietary principles to observe during treatment were presented at the time of first consult, but emphasized and discussed weekly during treatment visits, especially in those patients developing toxicity.
Toxicity is analyzed as both period and point prevalence. The former quantifies the proportion of patients with a given peak level of toxicity during the whole course of radiotherapy (from week 1 to week 8 of treatment). The latter describes the proportion of patients with a given peak level of toxicity at a specific time or week of treatment. Comparison of proportions between groups is done with a χ2 test unless otherwise specified; comparison of individual V-values was done with Wilcoxon matched-pairs t-test.
Results
Selected patient, tumor and treatment characteristics are summarized in . Detailed dosimetric data on PTVs coverage at planning are available for 82 of the 87 patients treated with WP-IMRT (). For five patients we were unable to restore the original plan. All patients had their PTV2 covered according to our criterion (V95% > 95%), while five patients (5/82, 6.1%) had a V95% for prostate PTV lower than 95%.
Table II. Selected patient, tumor and treatment characteristics.
illustrates the average planned DVH curves for the rectum, the bladder and the intestinal cavity for all the patients. For five patients with missing plan, data were reconstructed from the plan available in the treatment chart. Mean (±SD) doses to the rectum and bladder were 50.2±3.6 Gy and 51.8±4.6 Gy, respectively.
Overall 11 patients (11/82, 13.4%) failed to meet dose volume constraints for rectum/bladder and/or prostate PTV coverage ().
Table III. Details on 11 patients who failed to meet dose volume constraints and/or prostate PTV coverage.
Regarding clinical feasibility, the median delivered doses to PTV1 and PTV2 were 76 Gy (range: 70–78 Gy) and 54 Gy (range: 48.6–55.8 Gy), respectively, over 7.7 weeks (range: 7.0–11 weeks). Two patients experienced treatment breaks longer than 5 days, one due to acute toxicity (grade 3 proctitis) and one for logistic problems.
Mean V95 values taking into account the dose actually delivered were 97.6% (SD: 5.9%) and 98.2% (SD: 6.2%) for PTV1 and PTV2, respectively, and slightly reduced compared to planned values (). However, Wilcoxon matched-pairs t-test p-values between planned and delivered V95 were not significantly different at 0.22 and 0.25 for PTV1 and PTV2, respectively.
Overall, we collected an average of 7.9 evaluations for each of the ten acute toxicity items per patient during treatment (weeks 1 to 8).
No patient developed grade 4 acute toxicity. As summarized in , nine patients (10.3%) experienced peak grade 3 reactions involving one (6 patients) or two (3 patients) of the ten considered domains. Interestingly, all patients developing grade 3 toxicity had accompanying grade 2 toxicity in at least one (median 2, max 6) of the other GI/GU domains. Of the patients who failed to meet dose-volume objectives for the rectum and/or bladder, only one (7.1%) developed grade 3 toxicity.
Table IV. Peak prevalence of acute toxicity during treatment and at 3 months by domain.
About 2/3 of the patients developed grade 2 reactions as peak toxicity at some point during RT (). Reactions were equally distributed between the GI and GU domains. For patients developing peak grade 2 toxicity, the median number of domains with grade 2 toxicity was 2 (range: 1–5).
As detailed in , frequency/urgency and diarrhea were the most prevalent toxicities during the course of treatment (prevalence for grade 2+: 37.9% and 36.7%, respectively) followed by proctitis (21.8%) and dysuria (16.1%). None of the remaining items exceeded 10%.
Point prevalence of grade 2+ reactions is illustrated in for items with a meaningful number of events. Interestingly, for each selected GU domain, the proportion of patients with grade 2+ reactions is the largest during the last week of treatment; on the contrary, for GI domains, the proportion of patients with grade 2+ toxicity does not increase progressively during treatment but plateaus around the 4–5th week of treatment.
Figure 3. Point prevalence of grade 2+ toxicity by selected domains. Abbreviations: RB: rectal bleeding; F/U: frequency/urgency; UR: urinary retention.
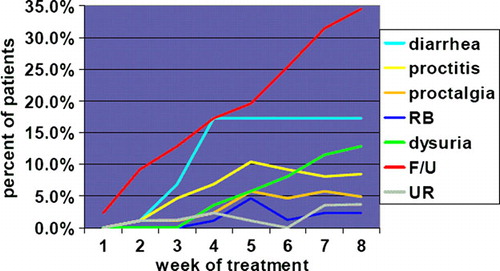
Moreover, peak point prevalence for both frequency/urgency and dysuria () approached their period prevalence (); on the contrary, peak point prevalence for diarrhea (17.2%) and proctitis (10.3%) is about half and significantly lower than period prevalence (36.8% and 21.8%, p < 0.01 and 0.04, respectively). Overall, the two features (timing and point/period prevalence comparison) are consistent with a progressive accumulation of GU reactions and with a short duration of GI ones during treatment.
Regarding the status of grade 2+ toxicity at the first follow up after treatment end, of all grade 2+ events during treatment for whom we have information at 3 months (115 events), 95 (82.6%) showed a complete resolution (or resolution to grade 0) at first follow up. Interestingly, such an improvement was unevenly distributed among domains: for grade 2+ GI domains complete resolution occurred in 62/67 cases (92.5%), while for GU only in 33/48 cases (68.7%), p = 0.001.
Discussion
The present paper was undertaken to satisfy all aims that justify the study of normal tissue side effects Citation[17]: 1. to serve as an integral part of our quality assurance program; 2. to describe the type, incidence and severity of side effects of WP-IMRT that are crucial for proper patient counseling; 3. to investigate the pathobiology underlying these effects. Regarding the second point, we calculated the prevalence rather than the incidence for two reasons: firstly, detailed information on pre-treatment toxicity was available only for two items; therefore, we cannot exclude that a symptom was present before starting treatment; secondly, the analysis of point prevalence over time would provide information on the duration of a specific side effect.
In the present paper, we confirm that WP-IMRT is dosimetrically feasible in most of the patients: we were unable to meet simultaneously PTV and OAR dose objectives only in 11 of 82 patients (13.4%). Violations were usually of small amplitude and without implications on acute toxicity ( and ). It remains to be determined whether they will have any impact on late toxicity.
Compared to PORT, WPRT with 4FBT has been recently associated with a statistically significant increase of both GI and GU grade 2+ side effects Citation[3]. We and others have shown the dosimetric advantage of WP-IMRT over WP RT with 4FBT for every OAR considered and without negative impact on target coverage Citation[5–7], Citation[18].
If the present results are compared to those obtained in 38 patients treated with IMRT to the prostate (76 Gy) +seminal vesicles (54 Gy) only (PORT + SV) during the same time frame at our institution and scored prospectively with the same approach, only grade 2+ diarrhea was significantly different between the two groups (WP-IMRT and PORT + SV) [unpublished data]. With PORT + SV, grade 2 period prevalence of diarrhea was 15.8% and none of the patients developed grade 3 diarrhea, compared to 36.7% (grades 2 and 3, ) for patients treated with WP-IMRT (p = 0.03). It is noteworthy to consider that the intestinal cavity dose volume constraints for most WP-IMRT plans had been based on the results obtained in the first 24 patients and/or they may have not been adequately stressed. The difference in diarrhea score between WP-IMRT and PORT + SV patients will be further investigated taking into consideration individual dose distribution data to the intestinal cavity. The intent is to assess whether we can derive more effective dose volume constraints and whether they can be successfully applied while respecting target(s) coverage. Moreover, dietary counseling was stressed only after the development of toxicity and this may have contributed to increase the proportion of patients with diarrhea during treatment. Liu et al. report a remarkably low (∼6%) prevalence of grade 2–3 toxicity during whole pelvic irradiation with standard 4FBT to 45 Gy at 1.8 Gy Citation[19]. Another factor that should be taken into consideration is that, in our study, a single episode of diarrhea was enough to register it as event contrary to other authors Citation[20].
Even if the proportion of patients experiencing grade 2 diarrhea at least once during treatment is higher than with PORT + SV, more than 80% of patients were free of grade 2+ diarrhea at any time during treatment, episodes were short lasting and most of the patients were symptom-free at 3 months after treatment end. Even if we cannot exclude that the results can be further optimized by more ‘aggressive‘ dosimetric and dietary approaches, we consider the above results remarkable in light of the dose delivered to the pelvic nodes.
Regarding rectal and bladder acute toxicity rates, as previously mentioned, they were not different from those obtained by PORT + SV. For the rectum, this is also consistent with the fact that the mean dose (50.2 Gy) delivered here is similar to the one (51 Gy) reported in a series that included mostly patients treated with conformal radiotherapy on the prostate + SV to a mean dose of 74 Gy (ICRU point)Citation[21]. Our data show that peak prevalence of grade 2+ proctitis was slightly higher than 10%, with most reactions subsiding by 3 months.
Contrary to GI reactions and to previous papers Citation[19], Citation[22], each selected GU item showed a progressive increase in prevalence during treatment rather than a plateau at mid-treatment. Moreover, grade 2+ GU events were significantly less likely to be fully resolved at 3 months compared to GI ones. A lack of benefit of IMRT over 3DCRT in reducing acute GU toxicity despite a better bladder dosimetry has been reported by Zelefsky et al. Citation[20]. This is consistent with the fact that urethritis and thus the dose to the prostate gland are the key factors rather than bladder sparing. Similarly, as suggested in the brachytherapy literature Citation[23], acute GU symptoms tend to last longer than 3 months, justifying the extension of acute toxicity definition to 9 months in one recent protocol Citation[24].
The present study has some limitations. First, ideally to properly assess the benefits of WP-IMRT we should have had a control group of patients treated to the same volumes and doses but with conformal radiotherapy instead of WP-IMRT. However, we had previously shown that whole-pelvis 4FBT cannot be considered iso-effective to WP-IMRT since underdosing of portions of PN PTV would take place in >¾ of patients Citation[9]. Two recent studies attempted such a comparison with conflicting acute toxicity results. In the first one, Ashaman et al. noticed a lower rate of acute GI toxicity in 13 patients treated with WP-IMRT compared to the one in 14 patients treated with a standard conformal approach. The difference was less evident for GU toxicity Citation[11]. Conversely, Jani et al. reported a reduction in GU but not in GI acute toxicity with WP-IMRT over whole-pelvis RT with 4FBT Citation[12]. Of note, the PN prescription dose was 45 Gy and up to 50.4 Gy for Jani et al. and Ashaman et al. papers, respectively, and comprehensive coverage of all pelvic nodal stations was not pursued in either one Citation[11], Citation[12]. While there are clinical data suggesting that comprehensive nodal coverage in this patients population is desirable Citation[2], we provide evidence that our approach, that targets all pelvic lymph node stations at risk, is both dosimetrically and clinically feasible in a large cohort of patients.
Second, there were eight different observers. Though some items/grades of CTC v2.0 are objectively defined, others represent a subjective interpretation (). However, while multiple observers cannot be avoided in routine practice, it should be noted that in the hands of RTOG researchers, the CTC v2.0 scale was easier to interpret than the RTOG one Citation[25]. Third, our observation time has a 3 month gap after treatment completion, and, looking at , we cannot exclude that the proportion of patients developing grade 2+ GU toxicity could have kept increasing after treatment end. In one series, one out of eight grade 3 GU events occurred after treatment completion Citation[26]. On the contrary in the Dutch trial more than 97% of patients developed grade 2+ toxicity within the first 7 weeks of treatment Citation[22].
Fourth, in 2003, CTC v2.0 was replaced by CTCAE v3.0. While we do not think that this is a real limitation since CTC 2.0 was specifically designed to score acute toxicity and CTCAE v3.0 was introduced to address other issues Citation[27], the two systems have important differences. However, as in the case of shift from the RTOG to the CTC v2.0 scale, the introduction of CTCAE v3.0 was done with the intent to preserve the severity scaling of previous systems Citation[28]. Fifth, we did not consider drug intake in the analysis, because it is recognized that over-the-counter drugs are often self-administered and may escape documentation Citation[26]. Finally, one may argue that optimal use of IMRT in this setting would include a simultaneous integrated prostate boost rather than a sequential approach Citation[29]. Our strategy has the advantage of avoiding the use of an unconventional (>2 Gy) dose per fraction with all the corresponding issues, including the uncertainties of scaling dose volume constraints and the use of hypofractionation whose benefit is not established for prostate cancer Citation[30]. A similar sequential approach has been also adopted by RTOG in protocol 0521 Citation[10]. It is remarkable that in our experience only about ¼ of patients needed two IMRT plans, while for the majority of patients WP-IMRT with a conformal 3D prostate boost provided an acceptable plan. As shown in , most (72.7%) of patients missing at least one of the planning objectives had been planned with a conformal boost. We felt that the limited amount of violation from dose objectives ( and ) along with the individual clinical context was not worth the use of IMRT for the boost plan. Accepting a more liberal use of IMRT for any case not meeting planning objectives with 3DCRT boost, still about 2/3 of patients would not have needed an IMRT boost.
Ultimately only three patients failed to meet dose/volume constraints after WP-IMRT and an IMRT boost (). The three cases share the features of missing multiple bladder dose-volume objectives and of having a significantly smaller than average bladder volume at simulation (Wilcoxon two sample test, p = 0.046). Since bladder volume at simulation does not necessarily represent the size of the organ during treatment Citation[31] and a small increase in filling would ‘bring down‘ the bladder DVH within the desired range, we felt that the plan was acceptable. However, in two other recent cases, patients with smaller bladder volumes at planning CT had been rescanned after supplemental instructions and dose objectives were formally met.
In conclusion, WP-IMRT for localized prostate cancer is dosimetrically and clinically feasible, though a high proportion of patients will experience peak grade 2+ acute toxicity at some point during treatment. However, we show that most of acute toxicity, especially GI, disappears by 3 months after treatment completion. In another paper we have shown that the preliminary rate of subacute rectal toxicity of WP-IMRT compares favorably with the one obtained with 3DCRT to the prostate alone Citation[32]. Further follow-up will clarify whether this approach is also feasible in terms of late complications.
References
- Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003; 21: 1904–11
- Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole-pelvic (WP) to prostate only (PO) radiotherapy and neoadjuvant to adjuvant total androgen suppression (TAS): Updated analysis of RTOG 94-13. Int J Radiat Oncol Biol Phys 2005;63:S19 [abstract].
- Roach M, 3rd, DeSilvio M, Valicenti R, et al. Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys 2006; 66: 647–53
- Garg AK, Mai WY, McGary JE, et al. Radiation proctopathy in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 1294–305
- Cavey ML, Bayouth JE, Colman M, et al IMRT to treat the pelvic nodes while escalating the dose to the prostate. Strahlether Onkol 2005 (accepted for publication).
- Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2004; 59: 267–84
- Nutting CM, Convery DJ, Cosgrove VP, et al. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys 2000; 48: 649–56
- Wang-Chesebro A, Xia P, Coleman J, et al. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 654–62
- Sanguineti G, Cavey ML, Endres EJ, et al. Is IMRT needed to spare the rectum when pelvic lymph nodes are part of the initial treatment volume for prostate cancer?. Int J Radiat Oncol Biol Phys 2006; 64: 151–60
- R.T.O.G. protocol 0521: A phase III protocol of androgen suppression (As) and 3DCRT/IMRT vs As and 3DCRT/IMRT followed by chemotherapy with docetaxel and prednisone for localized, high-risk prostate cancer. www.rtog.org.
- Ashman JB, Zelefsky MJ, Hunt MS, et al. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2005; 63: 765–71
- Jani AB, Su A, Milano MT. Intensity-modulated versus conventional pelvic radiotherapy for prostate cancer: Analysis of acute toxicity. Urology 2006; 67: 147–51
- Roach M, 3rd, Marquez C, Yuo HS, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 1994; 28: 33–7
- Fiorino C, Vavassori V, Sanguineti G, et al. Rectum contouring variability in patients treated for prostate cancer: Impact on rectum dose-volume histograms and normal tissue complication probability. Radiother Oncol 2002; 63: 249–55
- Sanguineti G, Culp L, Pena J, et al. Acute small bowel and colon toxicity after pelvic IMRT for prostate cancer. Eur J Cancer Suppl 2003; 1: S268
- Sanguineti G, Culp LR, Endres EJ, et al. Are neck nodal volumes drawn on CT slices covered by standard three-field technique?. Int J Radiat Oncol Biol Phys 2004; 59: 725–42
- Bentzen SM, Dorr W, Anscher MS, et al. Normal tissue effects: Reporting and analysis. Semin Radiat Oncol 2003; 13: 189–202
- Buyyounouski MK, Horwitz EM, Price RA, et al. Intensity-modulated radiotherapy with MRI simulation to reduce doses received by erectile tissue during prostate cancer treatment. Int J Radiat Oncol Biol Phys 2004; 58: 743–9
- Liu L, Glicksman AS, Coachman N, et al. Low acute gastrointestinal and genitourinary toxicities in whole pelvic irradiation of prostate cancer. Int J Radiat Oncol Biol Phys 1997; 38: 65–71
- Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002; 53: 1111–6
- Vavassori V, Fiorino C, Rancati T, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: Results of a prospective multicenter study. Int J Radiat Oncol Biol Phys 2007; 67: 1401–10
- Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 2005; 61: 1019–34
- Gelblum DY, Potters L, Ashley R, et al. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys 1999; 45: 59–67
- Lee WR, DeSilvio M, Lawton C, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: Preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys 2006; 64: 804–9
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol 2003; 66: 253–62
- De Meerleer G, Vakaet L, Meersschout S, et al. Intensity-modulated radiotherapy as primary treatment for prostate cancer: Acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys 2004; 60: 777–87
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–81
- Trotti A. The evolution and application of toxicity criteria. Semin Radiat Oncol 2002; 12: 1–3
- Bos LJ, Damen EM, de Boer RW, et al. Reduction of rectal dose by integration of the boost in the large-field treatment plan for prostate irradiation. Int J Radiat Oncol Biol Phys 2002; 52: 254–65
- Williams SG, Taylor JMG, Liu N, et al. Estimation of the a/ß ratio of prostate cancer using a multivariate analysis of the individual fraction size data of 3756 patients. Int J Radiat Oncol Biol Phys 2006; 66: S10–11
- Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys 1998; 41: 83–92
- Sanguineti G, Cavey ML, Endres EJ, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy?. Strahlenther Onkol 2006; 182: 543–9