Abstract
Background. The optimal care for patients with unresectable, non-metastatic pancreatic adenocarcinoma (PAC) is debated. We treated 17 consecutive cases with preoperative radiochemotherapy (RCT) as a means for downstaging their tumours and compared outcome with 35 patients undergoing direct surgery for primarily resectable PAC during the same time period. Methods. The patients had biopsy proven, unresectable, non-metastatic PAC which engaged ≥50% of the circumference of a patent mesenteric/portal vein for a distance ≥2 cm and/or <50% of the circumference of a central artery for <2 cm. The preop therapy included two courses of Xelox (oxaliplatin 130 mg/m2 d1; capecitabine 2 000 mg/m2 d1–14 q 3 w) followed by 3-D conformal radiotherapy (50.4 Gy; 1.8 Gy fractions) with reduced Xelox (d1-5 q 1 w X 6). Results. No incident of RCT-related CTC Grade 3–4 haematologic and six cases of non-haematologic side-effects were diagnosed. Sixteen patients completed the RCT and were rescanned with CT and reevaluated for surgery 4 weeks post-RCT. Five cases were diagnosed with new metastases to the liver. Eleven patients were accepted for surgery whereof eight underwent a curative R0-resection. The median overall survival for the latter group was 29 months, which compared favourably with our control group of patients undergoing direct curative surgery for primarily resectable PAC (median OS: 16 months; RO-rate: 75%). Perioperative morbidity was similar in the two cohorts but the duration of surgery was longer (576 vs. 477 min) and the op blood loss was greater (3288 vs. 1460 ml) in the RCT-cohort (p < 0.05). The 30-day mortality was zero in both groups. Conclusion. Preoperative RCT in patients with locally advanced PAC resulted in a high rate of curative resections and promising median survival in our treatment series. This trimodality approach merits further exploration in new studies, which are currently underway at our Department.
The optimal care for patients with unresectable, non-metastatic pancreatic ductal adenocarcinoma (PAC) is still debated Citation[1]. Advances in radiology, e.g. improvements in the quality of computed tomography (CT) scanning and magnetic resonance imaging (MRI), make it possible to diagnose tumour involvement of regional blood vessels with great accuracy in the preoperative setting Citation[2–5]. This minimizes the number of surgical explorations in patients with primarily unresectable, locally advanced PAC Citation[6].
Presently, radiochemotherapy is used extensively in the USA as an adjunct to surgery in resected patients and as a palliative treatment in cases with inoperable, locally advanced PAC. Radiochemotherapy is, however, less frequently used in Europe. This disagreement of opinions on the efficacy of bimodality therapy stems from conflicting results from several adjuvant trials Citation[7–10]. Furthermore, there are no data from randomised controlled trials supporting the superiority of radiochemotherapy in terms of survival or palliation compared with systemic chemotherapy alone in unresectable, locally advanced PAC Citation[11]. The possible benefit of radiochemotherapy as a preoperative strategy with the aim of downsizing primarily unresectable PAC is, however, not studied extensively Citation[12].
Long-term survival in patients with resected PAC relies heavily not only on T- and N-stage but also on R0-status, i.e. if the tumour is both macro- and microscopically radically resected Citation[13].
We treated 17 consecutive cases with locally advanced, unresectable PAC with preoperative radiochemotherapy as a means for downstaging their tumours with the aim at performing delayed R0-surgery and compared the outcome with 35 patients undergoing direct surgery for primarily resectable PAC during the same time period.
Material and methods
The patients who received preoperative radiochemotherapy were treated according to a national phase II protocol, which was approved by the ethics committee in Lund. They had given written informed consent prior to enrolment.
Patient population
All consecutive patients with local pancreatic carcinoma in the County Council of Stockholm, which were evaluated at the Karolinska multidisciplinary therapy (MDT) conference during 2002–4, were included in this analysis. The MDT-team included specialists in the field of advanced upper GI-surgery, -radiology, -oncology, and -pathology.
The patients were categorised as having primarily resectable (group A) or locally advanced, primarily unresectable tumours based on radiological criteria on CT, i.e. regional vascular invasiveness in the form of encasement of ≥50% of the circumference for a distance ≥2 cm. The latter group was further divided into possible surgical candidates following a downsizing effect of radiochemotherapy (group B), i.e. tumour involvement of a patent portal or mesenteric vein as described previously and/or regional arterial encasement of <50% for a distance <2 cm, or definitively unresectable cases, i.e. more advanced regional vascular involvement (group C). The used radiological, presurgical categorisation is summarized in .
Table I. Radiological categorisation of pancreatic tumours (A-C) according to regional vessel involvement.
All primarily unresectable cases underwent a fine needle biopsy for a definitive diagnosis of PAC prior to therapy. Patients included in group C were recommended palliative gemcitabine-based chemotherapy alone as the standard of care and they were not included in the following analyses. Patients in group B were invited to take part in a prospective phase II study, which tested the efficacy of radiochemotherapy in locally advanced PAC. Characteristics for this group of patients (B; n = 17), group A patients who underwent resection with curative intent during surgical exploration (A 1: n = 29), and group A patients who were not resected due to peroperative signs of locally advanced disease (A 2: n = 6) are shown in .
Table II. Demographic data for the three patient cohorts, i.e. A1–2, B1,2,3.
Radiochemotherapy regimen
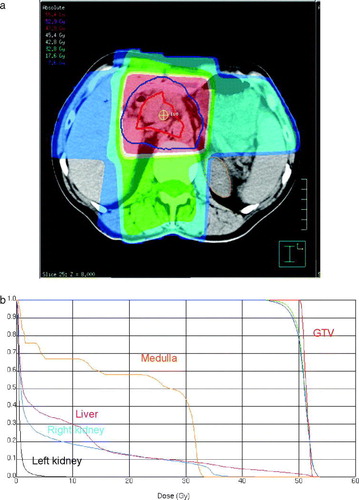
The preoperative chemoradiation therapy consisted of two courses of Xelox (oxaliplatin 130 mg/m2 iv d1; capecitabine 1000 mg/m2×2 po d1–14 q 3 w) followed by 3 dimensional conformal radiotherapy (50.4 Gy; 1.8 Gy fractions) in combination with a reduced Xelox regimen (oxaliplatin 30 mg/m2 iv d1 or d2; capecitabine 675 mg/m2×2 po d1–5 q 1 w X 6). An example of a typical radiation field arrangement and dose distribution is shown in A. Organs at risk for radiation-induced side effects were contoured on the dose planning CT and dose volume histograms (DVH) for normal tissues, viz. the medulla, liver and kidneys, and gross tumour volume (GTV) were calculated. An example of representative DVHs for the former and latter metrics are shown in B.
Figure 1. a: 3 dimensional radiotherapy dose plan. The red and the blue lines depict the gross tumour volume (GTV) and clinical target volume, respectively. The red area marks 95% of the prescribed dose. b: Cumulative dose volume histograms for the GTV and organs at risk.
Patients were recommended 5-HT3-antagonist antiemetics prior to every radiotherapy treatment and they were generally on PPI-therapy during the entire radiochemotherapy period. The patient's weight and well-being was, furthermore, checked weekly by an experienced GI-radiotherapist.
Radiological pre- and post-therapy assessments
The categorization of patients was based on a preoperative, dynamic computed tomography (CT) scan of the upper abdomen according to our standard protocol. Thirty min before scanning, the patients consumed 1 L of water as a non-opaque intraluminal contrast agent. The examinations were obtained with a four-detector CT scanner (Somatom Volume Zoom; Siemens Medical, Munich, Germany) using a dual phase pancreatic protocol. Initially, an un-enhanced scan was obtained using about 15 s breathhold acquisition, 2.5 mm collimation, and a pitch of 1.5. Intravenously, 150 mL of iodixanol 320 mg I/mL (Visipaque®, GE Healthcare, Little Chalfont, UK) was administered at a rate of 4 mL/sec with a Mallinckrodt CT 9000® ADV injector (Hazelwood, MO, USA). To acquire arterio-portal and portal-venous phases bolus tracking was used (triggering at 100 Hounsfield units in the aorta; delay 15 and 70 s, respectively). The slice collimation for both contrast phases was 1 mm and the pitch was 1.5. They were obtained during breathhold (10–15 s and 15–20 s, respectively). Images were reconstructed in the axial, sagittal, and coronal planes (3 mm for the arterial-portal phase and 5 mm for the portal-venous phase). The arterial-portal phase covered the entire liver and the pancreas whereas the portal-venous phase covered the area from the diaphragm to the symphysis pubis. The preoperative staging included an MRI of the upper abdomen in unclear cases, when radiological resectability could not be determined by CT alone. The liver was carefully screened for signs of metastases in order to exclude cases of early systemic disease and the preoperative staging also included a CT of the thorax to detect lung metastases. In uncertain cases of metastatic disease, whole body FDG-PET or contrast enhanced ultrasonography of the liver was included in the preoperative screening. The mean maximum tumour diameter among the patients who were selected for downstaging radiochemotherapy (group B) was 34 mm (SE±2). In 16 of these cases, the length of the vascular involvement was ≥20 mm and in 16 patients it engaged ≥50% of the circumference of a patent regional vein or artery. Fifteen cases had both radiological signs of irresectability at base line.
Patients undergoing preoperative radiochemotherapy (group B) were rescanned 4 weeks after the cessation of therapy and reevaluated for surgery by our MDT-team. Criteria for surgical exploration following preoperative therapy included definitive signs of response in the form of tumour shrinkage and decrease of vascular engagement.
Surgical techniques
A standard pancreato-duodenectomy Citation[14], Citation[15] was performed, which included a distal gastrectomy and a retroperitoneal dissection, harvesting Japanese Pancreas Society lymph node stations 16a2 and 16b1 and sampling lymph node station 9. There was no circumferential clearance of the coeliac axis or the superior mesenteric artery, but there was complete nodal harvesting from the hepatoduodenal ligament and the common hepatic artery. Resection of the portal or superior mesenteric vein was performed in eight patients to achieve an R0-resection (four cases in group A1 and B, respectively). In one patient, resection of the hepatic artery with end-to-end anastomosis without grafting was performed. Reconstruction of the pancreatic-enteric anastomosis was done with an end-to-side duct-to-mucosa technique, without a pancreatic stent.
After removal of the specimen, the resection margins at the transected pancreas, hepatic duct, and posterior surface at the superior mesenteric artery were carefully sampled to assess R-stage.
Definition of postoperative morbidity
A pancreatic fistula was defined as >25 mL/d of amylase-rich fluid output by the abdominal drain for 8 days or after the 8th postoperative day. Registered surgical complications also included delayed gastric emptying, bleeding, and wound infection/dehiscence. Medical complications were defined as non-surgical events that prolonged in-hospital stay or led to active treatment.
Statistics
Comparison of medians was executed with the Mann-Whitney U-test (2-sided). Overall survival was estimated with the Kaplan-Meier actuarial method. Differences in survival were tested with the log-rank test. A p-value <0.05 was considered statistically significant.
Results
Efficacy
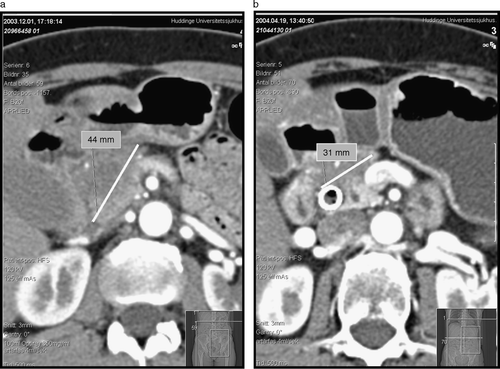
An example of the potential downsizing effect of preoperative radiochemotherapy is shown in A,B. Preoperative therapy resulted in clear tumour shrinkage and increased patency of the splenic vein in this individual. This patient is alive and without signs of recurrence 35 months following start of therapy.
Figure 2. Computed tomography scan of the upper abdomen depicting the size of the tumour before radiochemotherapy (a) and 4 weeks after the completion of therapy (b). Note the increased patency of the splenic vein following treatment.
Sixteen of the original 17 patients who started preoperative therapy finished their scheduled treatment (cohort B). Five of these patients had signs of liver metastases on their follow-up CT. Eleven cases were accepted for surgical exploration following downsizing preoperative radiochemotherapy and eight of theses underwent a resection with curative intent. Three individuals were still found to be unresectable despite the given preoperative treatment during explorative surgery.
Out of the 35 patients, who were judged as radiologically primarily resectable, six (cohort A2) were deemed unresectable during surgical exploration due to locally advanced disease not diagnosed by the preoperative radiological imaging.
Data on therapeutic efficacy for the cohorts (A1–2, B) are given in . An R0-situation was accomplished in all eight resected patients in the cohort which underwent preoperative radiochemotherapy (B), while the R0-rate was 75% in cases undergoing direct surgery for radiologically primarily resectable tumours (A1). Furthermore, survival curves for resected patients in the two cohorts are depicted in . The median survival and estimated 4-year survival for cohort B and A1 was 29 and 16 months and 24% and 11%, respectively (log-rank test p = 0.02). There was no statistical difference between the median survival for all patients in cohort A (n = 35; 11 months) and cohort B (n = 17; 19 months) (log-rank test p = 0.18).
Figure 3. Survival curves from start of therapy for patients undergoing direct surgery for PAC (solid line) compared to patients treated with preoperative radiochemotherapy and delayed resection due to radiological signs of unresectability at base line (dotted line).
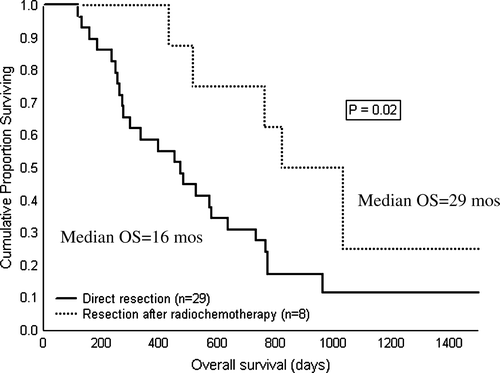
Table III. Surgical and morbidity data for the three patient cohorts, i.e. A1–2, B1,2,3.
Side effects/surgical morbidity
Six cases of non-haematological and no case of haematological CTC Grade 3–4 Citation[16] were observed during the radiochemotherapy period in our treatment series. The main problem during the preoperative period was stent dysfunction which required intervention in six cases, viz. replacement. There was one case of major loss of weight but no patient was hospitalised during the treatment period due to nutritional problems. However, three patients received i.v. supplements of calories/fluids in their homes. All patients were, furthermore, on weekly check-ups at our nutritionist and the majority supplemented their normal diets with nutritional drinks. One patient stopped treatment early due to failure to thrive symptoms and signs of depression, which could not be managed with medical treatment.
Rates of surgical and postoperative medical morbidity in the three studied cohorts (A1, A2, and B) are displayed in . The 30-day morbidity was zero in both cohorts, which underwent curative resections.
Discussion
Preoperative radiochemotherapy for radiologically locally advanced PAC and reevaluation for possible surgical exploration following a downsizing effect appeared to be a reasonable therapeutic strategy in our treatment series of 17 patients. The median survival of about 30 months and the high R0-rate for the subset of responding patients who underwent surgical resection following preoperative therapy compared favourably with outcome data for cases which underwent direct surgical exploration/resection for radiologically primarily resectable tumours during the same time period. Furthermore, the two therapeutic strategies, i.e. direct surgery for resectable disease (n = 35; 11 months) vs. preoperative radiochemotherapy for primarily unresectable tumours (n = 17; 19 months), appeared to yield similar median survival when all patients were considered in the analysis. A comparison between our results and the more recent, previous reports is displayed in Citation[17–25].
Table IV. Results from recent preoperative radiochemotherapy treatment series in pancreatic cancer.
In the majority of cases with PAC, i.e. 65–85% Citation[26], the tumour is not surgically resectable at diagnosis, evenly due to liver metastasis or local tumour aggressiveness, e.g. vessel engagement Citation[27]. The effectiveness of radiochemotherapy as definitive treatment or as preoperative therapy in locally advanced PAC has yet not been proven in randomised controlled clinical trials. In a small Japanese controlled trial by Shinchi and co-workers, the combination of radiochemotherapy and systemic treatment significantly improved survival in patients with locally advanced PAC compared to best supportive care alone Citation[28]. However, at last year's ASCO, Chauffert et al. reported no survival benefit for radiochemotherapy compared to systemic gemcitabine in patients with grossly unresectable tumours Citation[11]. It should be noted that the latter category of patients was not included in our study which focused on younger, fit patients with smaller tumours, deemed potentially resectable following a therapeutic downsizing effect. We do, however, stress that the extended median survival in our cohort which underwent radiochemotherapy and resection in part is due to patient selection, as the cases that developed liver metastases during therapy are excluded from the analysis in . Thus, patients with tumours with aggressive biology were singled out during the radiochemotherapy period due to early systemic progression.
It is important to note that we achieved an R0-status in all patients who underwent resection following preoperative therapy and that this probably partly explains the extended median survival in this cohort. We also stress that the radiotherapy target volumes were comparatively small in this series and that the patients were carefully monitored to avoid weight loss during the treatment period.
Despite good quality preoperative radiological imaging, roughly 15% of cases in this series were inaccurately categorized as resectable and unnecessarily surgically explored. This rate is similar to the finding of a recently reported series by Karmazanovsky et al. Citation[2] but lower than older studies Citation[3], Citation[5].
The survival curves for the patients undergoing direct surgery and curative resection for radiologically resectable disease in our series () is similar to data obtained in the ESPAC-I trial in the surgery alone arm Citation[7]. Postoperative, adjuvant chemotherapy following R0–1 resections extends survival in patients with primarily resectable PAC Citation[7], Citation[29]. Whether preoperative, neoadjuvant radiochemotherapy is better than direct surgery in patients with primarily resectable PAC is presently tested in an ongoing German randomised controlled trial. The difference in median survival between our two cohorts which underwent resection () implies that preoperative radiochemotherapy could be an alternative also in patients with primarily resectable disease. Furthermore, the lack of difference in median survival of the two cohorts when all patients were considered in the survival analysis (direct surgery: 11 months vs. preoperative radiochemotherapy: 19 months), suggests that neoadjuvant radiochemotherapy could safely be studied also in patients with primarily resectable tumours.
In conclusion, we found that preoperative radiochemotherapy and delayed resection in younger, fit patients with radiologically unresectable PAC due to moderate regional vessel tumour involvement was a successful strategy and that the outcome for these patients compared favourably with the control group of patients who underwent direct surgery for radiologically resectable tumours. We consider this topic worthy further research, preferably in the setting of randomised controlled trials, which is currently underway at our Department.
This paper was presented at GI ASCO 2007 Orlando, Florida USA.
References
- Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer?. J Gastrointest Surg 2002; 6: 763–9
- Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: Accuracy of CT in determination of resectability. Abdom Imaging 2005; 30: 488–500
- House MG, Yeo CJ, Cameron JL, Campbell KA, Schulick RD, Leach SD, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg 2004; 8: 280–8
- Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB, Jr. MDCT in Pancreatic adenocarcinoma: Prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol 2004; 182: 419–25
- Valls C, Andia E, Sanchez A, Fabregat J, Pozuelo O, Quintero JC, et al. Dual-phase helical CT of pancreatic adenocarcinoma: Assessment of resectability before surgery. AJR Am J Roentgenol 2002; 178: 821–6
- McCarthy MJ, Evans J, Sagar G, Neoptolemos JP. Prediction of resectability of pancreatic malignancy by computed tomography. Br J Surg 1998; 85: 320–5
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200–10
- Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776–82; discussion 82–4.
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899–903
- Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 1987;59:2006–10.
- Chauffert B, Mornex F, Bonnetain F, Triboulet J, Bouche O, Rougier P, et al. Phase III trial comparing initial chemoradiotherapy (intermittent cisplatin and infusional 5-FU) followed by gemcitabine vs. gemcitabine alone in patients with locally advanced non metastatic pancreatic cancer: A FFCD-SFRO study. J Clin Oncol 2006; 24: 180s, (Abstr:4008)
- Krempien R, Muenter MW, Harms W, Debus J. Neoadjuvant chemoradiation in patients with pancreatic adenocarcinoma. HPB 2006; 8: 22–8
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann Surg 1997;226:248–57; discussion 57–60.
- Jones L, Russell C, Mosca F, Boggi U, Sutton R, Slavin J, et al. Standard Kausch-Whipple pancreatoduodenectomy. Dig Surg 1999; 16: 297–304
- Pedrazzoli S, Beger HG, Obertop H, Andren-Sandberg A, Fernandez-Cruz L, Henne-Bruns D, et al. A surgical and pathological based classification of resective treatment of pancreatic cancer. Summary of an international workshop on surgical procedures in pancreatic cancer. Dig Surg 1999; 16: 337–45
- Cancer Therapy Evaluation Program. Common toxicity criteria, Version 2.0 DCTD, NCI, DHHS. Accessable at: http://ctep.cancer.gov.
- Wanebo HJ, Glicksman AS, Vezeridis MP, Clark J, Tibbetts L, Koness RJ, et al. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg 2000;135:81–7; Discussion 8.
- Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer 2000; 89: 314–27
- White RR, Hurwitz HI, Morse MA, Lee C, Anscher MS, Paulson EK, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol 2001; 8: 758–65
- Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001; 5: 27–35
- Wilkowski R, Thoma M, Heinemann V, Rau HG, Wagner A, Stoffregen C, et al. Radiochemotherapy with gemcitabine and cisplatin in pancreatic cancer – feasible and effective. Strahlenther Onkol 2003; 179: 78–86
- Magnin V, Moutardier V, Giovannini MH, Lelong B, Giovannini M, Viret F, et al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys 2003; 55: 1300–4
- Aristu J, Canon R, Pardo F, Martinez-Monge R, Martin-Algarra S, Manuel Ordonez J, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol 2003; 26: 30–6
- Calvo FA, Matute R, Garcia-Sabrido JL, Gomez-Espi M, Martinez NE, Lozano MA, et al. Neoadjuvant chemoradiation with tegafur in cancer of the pancreas: Initial analysis of clinical tolerance and outcome. Am J Clin Oncol 2004; 27: 343–9
- Joensuu TK, Kiviluoto T, Karkkainen P, Vento P, Kivisaari L, Tenhunen M, et al. Phase I–II trial of twice-weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004; 60: 444–52
- Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: A 20-year experience in 516 patients. Arch Surg 2004;139:718–25; Discussion 25–7.
- Trede M, Richter A, Wendl K. Personal observations, opinions, and approaches to cancer of the pancreas and the periampullary area. Surg Clin North Am 2001; 81: 595–610
- Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2002; 53: 146–50
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007; 297: 267–77