To the Editor
The WHO-EORTC classification divides cutaneous B-cell lymphomas into four categories of which primary cutaneous diffuse large B-cell lymphoma, leg type (PCLBCL, leg type) characteristically involves lower legs of elderly women and shows a predominance of confluent sheets of medium sized to large cells with round nuclei and prominent nucleoli resembling centroblasts and/ or immunoblasts. The neoplastic B-cells of PCLBCL, leg type have characteristic immunophenotype which readily differentiates them from other subtypes of primary cutaneous diffuse large B-cell lymphomas. The overall prognosis in this group is poor with a tendency to extracutaneous dissemination. An association of PCLBCL, leg type with Epstein Barr virus (EBV) has not been previously described. We report an unusual case of PCLBCL with immunophenotypic features of leg type with EBV positivity in the neoplastic B-cells by in situ hybridization. A fifty-five year old Caucasian male presented with a subcutaneous nodule on his right thigh which was completely excised. Imaging studies did not reveal any systemic disease. Staging bone marrow biopsy performed at that time was negative. Past medical history is significant for hypertension, diabetes mellitus type 2, hyperlipidemia, cerebrovascular accident in 2001 and 2005 for which he received anticoagulant therapy and developed a skin rash.
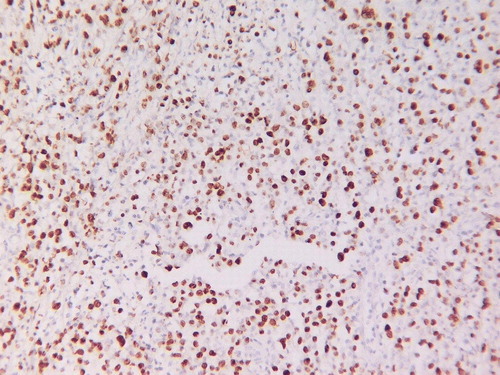
The nodule that was recently excised measured 1–1.5 cm in diameter and sections show focal skin ulceration with areas of necrosis and a dense lymphoid infiltrate in the dermis extending into superficial subcutaneous tissue. The neoplastic lymphoid infiltrate is composed of large cells with vesicular chromatin with single to several nucleoli resembling centroblasts and immunoblasts. Scattered apoptotic bodies, mitotic figures and pleomorphic cells are noted. () There is a dense reactive T-cell infiltrate. The neoplastic lymphoid cells and pleomorphic cells are B-cells expressing CD20 () and Bcl-2 () with focal positivity for CD30. They are negative for CD79a, CD10, CD15, cyclinD1 and ALK1. Proliferative index marker (Ki-67) is high (60%). CD3, CD4, CD5, CD7, CD8 and CD45RO highlight background reactive T-cells. These immunophenotypic and histologic findings are consistent with PCLBCL, leg type according to the WHO-EORTC classification. Surprisingly, in situ hybridization for Epstein Barr virus (EBV) was positive ().
Figure 1. H and E stained section showing the neoplastic lymphoid infiltrate composed of large cells with vesicular chromatin and single to several nucleoli resembling centroblasts and immunoblasts. Scattered apoptotic bodies, mitotic figures and pleomorphic cells are noted (original magnification×400).
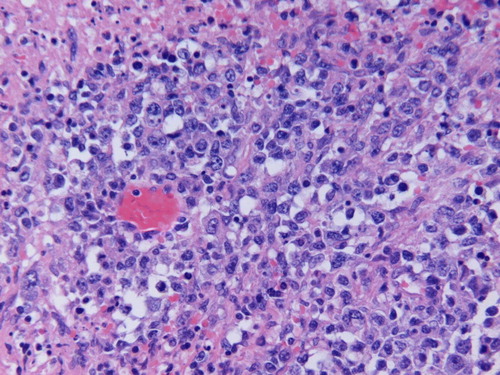
Figure 2. CD20 immunostain demonstrates the neoplastic infiltrate to be B-cells (original magnification×400).
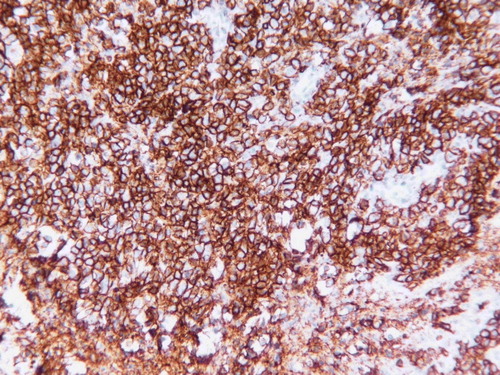
Figure 4. EBV in situ hybridization demonstrates positivity in the neoplastic large B-cells (original magnification×400).
Our patient refused chemotherapy and about six months since diagnosis, has no evidence of local recurrence or extracutaneous disease for which he is closely followed up.
Discussion
Primary cutaneous large B-cell lymphomas (PCLBCL) represent a heterogeneous group of B-cell neoplasms which present in the skin without evidence of extracutaneous disease Citation[1], Citation[2]. Classification of PCLBCL has been a matter of ongoing debate. The European Organization for Research and Treatment of Cancer (EORTC) approach has been to subdivide PCLBCL on the basis of site to produce prognostically relevant groups. They distinguished two main groups of PCLBCL Citation[3]. Most cases fell in the group of primary cutaneous follicle center-cell lymphomas (PCFCLs) that were composed of centroblasts and centrocytes of various sizes and that were classified as either centroblastic/centrocytic or centroblastic lymphomas according to the criteria of the Kiel classification Citation[4]. These PCFCLs appeared as a well-defined group of primary cutaneous B-cell lymphomas which often presented with skin lesions confined to the head or the trunk and had an excellent prognosis irrespective of the proportion of large cells and histologic subclassification Citation[5]. However, it was noted from the first publications regarding these PCFCLs that patients presenting with skin tumors on the leg had a different clinical behavior Citation[5]. Since these tumors of the leg were consistently composed of a majority of large B-cells, the term PCLBCL, leg type was used Citation[6]. Recent studies have demonstrated that patients with PCLBCL, leg type differed from patients with PCFCL arising at other sites by an older age of onset, poorer prognosis, and the almost constant expression of bcl-2 protein Citation[7]. For these reasons, PCLBCL, leg type was included as a separate entity in the EORTC classification as an intermediate prognosis tumor on the leg Citation[3]. This entity was not recognized by the World Health Organization (WHO) classification in 2001 and it categorized the PCLBCL as a heterogeneous disorder encompassing several disease entities Citation[8]. The new WHO-EORTC consensus classification is a major improvement on the independent classifications and contributes significantly to a more uniform diagnosis and management of cutaneous lymphomas. It recognizes PCLBCL, leg type as a distinct entity with an unfavorable prognosis Citation[9]. Patients present with generally rapidly growing red or bluish-red tumors on one or both (lower) legs. Uncommonly, this lymphoma can arise at sites other than the legs. In contrast to the group of PCFCLs, they more often disseminate to extracutaneous sites Citation[10]. Histologically, these lymphomas show diffuse infiltrates of a monotonous population or sheets of centroblasts and immunoblasts Citation[6], Citation[10] which often extend into the subcutaneous tissue. Mitotic figures are frequent, small B-cells are lacking and reactive T-cells are relatively few and often confined to perivascular areas. Our case showed an unusual positivity for EBV in the neoplastic cell population. Kodama et al. Citation[11] analyzed 40 cases of PCLBCL, leg type for presence of infectious agents including HHV-8, EBV, and B. burgdorferi, but could find only one case of PCLBCL, leg type positive for HHV-8. HHV-8 is well known as the causative agent of Kaposi sarcoma Citation[12] and primary effusion lymphoma, which is a subtype of diffuse LBCL Citation[13]. No EBV positive cases were noted Citation[11]. A prominent stromal reaction as seen in PCFCLs is not observed. In contrast to the group of PCFCLs, PCLBCL, leg type, shows strong bcl-2 expression, also in cases not located on the legs Citation[14]. Bcl-6 is expressed by most cases, whereas CD10 staining is generally absent Citation[15]. Unlike PCFCL, most PCLBCL, leg type, express MUM-1/IRF4 protein Citation[16]. t(14;18) is not found in PCLBCL, leg type and the Bcl-2 over expression may result from chromosomal amplification of the bcl-2 gene in some cases Citation[17]. Recent studies using interphase FISH analysis demonstrated translocations involving myc, bcl-6, and IgH genes in 11 of 14 PCLBCL, leg type cases, but not in patients with a PCFCL with a diffuse infiltration of large centrocytes Citation[18].
We present an unusual case of PCLBCL clinically, morphologically and immunophenotypically consistent with leg type showing EBV positivity. The significance of this finding is elusive at the present time and may suggest an etiologic role in this case. The robust infiltrate of reactive T-cells may be in response to the EBV. The EORTC-WHO approach to PCLBCLs is to categorize them into three main groups: PCFCLs, PCLBCL, leg type and PCBCL, other; the latter being a wastebasket category to include “atypical” cases that do not fit in the other two groups. Studies suggest that cases falling in the PCLBCL, other category seem to have an intermediate prognosis. Including our case in the PCLBCL, other category due to EBV positivity may not be appropriate at this time. Our case reinforces the fact that the new WHO-EORTC classification is merely a beginning indicating a direction in which research in PCLBCLs needs to develop. New evidence from gene and protein expression profiling and other methodologies will result in a much needed further updated classification.
References
- Kerl H, Cerroni L. Primary B-cell lymphomas of the skin. Ann Oncol 1997; 8(suppl 2)29–32
- Pimpinelli N, Santucci M, Mori M, Vallecchi C, Giannotti B. Primary cutaneous B-cell lymphoma: A clinically homogeneous entity?. J Am Acad Dermatol 1997; 37: 1012–6
- Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: A proposal from the cutaneous lymphoma study group of the European Organization for Research and Treatment of Cancer. Blood 1997; 90: 354–71
- Stansfeld AG, Diebold J, Noel H, Kapanci Y, Rilke F, Kelenyi G, et al. Updated Kiel classification for lymphomas. Lancet 1998;1:292–3 (letter).
- Willemze R, Meijer CJLM, Scheffer E. Diffuse large cell lymphomas of follicle center cell origin presenting in the skin: A clinicopathologic and immunologic study of 16 patients. Am J Pathol 1987; 126: 325–33
- Vermeer MH, Geelen FAMJ, van Haselen CW. Primary cutaneous large B-cell lymphomas of the legs: A distinct type of cutaneous B-cell lymphoma with an intermediate prognosis. Arch Dermatol 1996; 132: 1304–08
- Grange F, Hedelin G, Joly P, Beylot-Barry M, D'Incan M, Delaunay M, et al. Prognostic factors in primary cutaneous lymphomas other than mycosis fungoides and the Sézary syndrome. Blood 1999; 93: 3637–42
- Jaffe ES, Harris NL, Stein H, Vardiman W, et al. World Health Organization classification of tumors: Pathology and genetics of tumours of hematopoietic and lymphoid tissues. LyonFrance: IARC Press; 2001.
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Review article. Blood 2005; 105: 3768–85
- Grange F, Bekkenk MW, Wechsler J, Meijer CJLM, Cerroni L, Bernengo M, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: A European multicenter study. J Clin Oncol 2001; 19: 3602–10
- Kodama K, Massone C, Chott A, Metze D, Kerl H, Cerroni L. Primary cutaneous large B-cell lymphomas: Clinicopathologic features, classification, and prognostic factors in a large series of patients. Blood 2005; 106: 2491–7
- Chang Y, Moore PS. Kaposi's Sarcoma (KS) – associated herpesvirus and its role in KS. Infect Agents Dis 1996; 5: 215–22
- Said JW, Tasaka T, Takeuchi S, Asou H, de Vos S, Cesarman E, et al. Primary effusion lymphoma in women: Report of two cases of Kaposi's sarcoma herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood 1996; 88: 3124–8
- Geelen FAMJ, Vermeer MH, Meijer CJLM, Van der Putte SCJ, Kerkhof E, Kluin PM, et al. Bcl-2 expression in primary cutaneous large B-cell lymphoma is site-related. J Clin Oncol 1998; 16: 2080–5
- Hoefnagel JJ, Vermeer MH, Janssen PM, Fleuren GJ, Meijer CJ, Willemze R. Bcl-2, Bcl-6 and CD10 expression in cutaneous B-cell lymphoma: Further support for a follicle centre cell origin and differential diagnostic significance. Br J Dermatol 2003; 149: 1183–91
- Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood 2005; 105: 3671–8
- Mao X, Lillington D, Child FJ, Russell-Jones R, Young B, Whittaker S. Comparative genomic hybridization analysis of primary cutaneous B-cell lymphomas: Identification of common genomic alterations in disease pathogenesis. Genes Chromosomes Cancer 2002; 35: 144–55
- Hallermann C, Kaune KM, Gesk S, Martin-Subero J.I. Molecular cytogenetic analysis of chromosomal breakpoints in the IGH, MYC, BCL6 and MALT1 gene loci in primary cutaneous B-cell lymphomas. J Invest Dermatol 2004; 123: 213–9