Abstract
Background and purpose. Elderly women with breast cancer are often not given adjuvant chemotherapy (CT). One reason for this is that older women are believed to have more problems in tolerating side-effects of CT. The purpose of this study was to analyze the impact of age on health related quality of life (HRQoL) and symptoms in postmenopausal women with breast cancer undergoing adjuvant CT. Patients and methods. Eighty consecutive postmenopausal patients planned for CT were invited. Seventy-five agreed to participate (age 55–77 years). The patients completed two cancer-specific HRQoL questionnaires, The European Organisation for Research and Treatment of cancer (EORTC) EORTC-QLQ-C30, the EORTC-QLQ-BR23, and the Hospital Anxiety and Depression Scale (HADS) before, during, and 4 months after completion of treatment. The design was descriptional and longitudinal. Correlations were examined between age and change in HRQoL variables. Results. No significant correlations were found between age and any of the assessed HRQoL domains or symptom scales, except for dyspnoea and sexual functioning. Age was inversely correlated to change in dyspnoea from baseline through follow-up, whereas older women perceived their sexual functioning significantly lower at baseline. Conclusion: The results indicate that among postmenopausal patients in the age range 55–77 years consecutively selected for adjuvant CT age was not a predictor of decreased HRQoL. This supports the argument that age should not be used in isolation in decisions about adjuvant CT for breast cancer in elderly women.
Cancer treatment of the elderly patient is an increasingly important concern since the risk for developing breast cancer increases with age. Of the approximately 6 900 women who developed the disease in Sweden during 2004, about 65% were postmenopausal and about 30% were aged 70 or older Citation[1]. The effects of adjuvant CT on the breast cancer disease seem to be as good for pre and postmenopausal patients, at least for the hormone receptor negative patients Citation[2]. However, very few patients older than 70 years were included in the randomised studies Citation[2].
In clinical practice, it is common that elderly women are offered less CT Citation[3], Citation[4], because of the fear that they are less able to tolerate toxicity Citation[5]. Nevertheless, some studies have shown that elderly patients tolerate standard chemotherapy regimes, and even more intensive regimes, almost as well as younger patients Citation[6].
Another aspect highly important is quality of life. Most studies concerning this aspect have been performed on the younger and middle-aged patients. These studies have shown that the patients experience a number of different symptoms related to both the treatment and/or the disease Citation[7], Citation[8]. These symptoms may also continue well after the treatment has ended Citation[9], and can lead to decreased health-related quality of life (HRQoL) Citation[10], Citation[11]. However, it is questionable whether findings from younger women can be accurately extrapolated to an elderly population Citation[12]. A greater understanding of age-related differences in experienced HRQoL and symptoms, in conjunction with chemotherapy is therefore an important issue for clinicians in oncology care Citation[13].
The purpose of this study was to analyze the impact of age on experienced HRQoL and symptoms in postmenopausal women with breast cancer before, during and 4 months after adjuvant CT. We defined HRQoL as a subjective perception of health, i.e. the impact of disease and treatment on health status (functions, symptoms and subjective well-being). This definition has also been advocated by the European Organisation for Research and Treatment of Cancer (EORTC) Citation[14].
Material and methods
Sample and setting
The inclusion criteria for this study were: postmenopausal women aged 55 years and older newly diagnosed with histologically confirmed stage I to IIIa breast carcinomas, and able to give informed consent, read and speak Swedish, and understand the purpose of the study. Age 55 years or above was used to ensure that the women participating in the study were homogeneous with respect to postmenopause. The age range was 55–77 years. The exclusion criteria were: evidence of dementia, known history of psychiatric disorder, and history of other cancers within the previous five years. The participants were consecutively invited patients who after prior surgery were scheduled for adjuvant chemotherapy treatment (CT) at two university hospitals and one county hospital in Sweden. Chemotherapy was offered to all patients with hormone receptor-negative breast cancer and to high-risk hormone receptor-positive patients (i.e. tumor size > 50mm and/or more than 3 positive lymph nodes). Many of the women also had the CT combined with RT (a 5-week radiotherapy course starting 3–4 weeks after CT), and/or endocrine therapy
Procedure
Data were collected from November 2003 to November 2005. Of 80 consecutive patients who met the inclusion criteria, only five refused participation (n = 75). The CT regimen was either flurouracil 600 mg/m2 i.v., epirubicin 75 mg/m2 i.v., and cyclophosphamide 600 mg/m2 i.v., (FEC) every third week in a course of six treatments (n = 72,), or cyclophosphamide 100 mg/m2 per orally on days 1–14 and methothrexate 40 mg/m2 i.v., and flurouracil 600 mg/m2 i.v. on days 1 and 8 (CMF) in a course of six treatments (n = 3).
A pilot study was performed with ten of the included 75 women about to receive CT in order to investigate whether there were any problems with the data collection method. No adjustments were found to be needed to the study design, instruments, or data collection procedure.
The baseline data were collected one week before treatment. The women from one of the university hospitals were interviewed and filled in the questionnaires either at the hospital or at home, whereas the women from the county hospital and the other university hospital were interviewed by telephone due to geographical distances. In the latter groups, questions and response choices were read to the women and their answers were filled in by the interviewer. At subsequent data collection points, i.e. one week after the first, third, and last cycles of chemotherapy, and at follow-up four months after the completion of treatment, all patients were mailed their questionnaires, together with an answer envelope and a letter explaining the procedures and providing the telephone number of the first author.
A total of 24% (n = 18, mean age 65 years) of the original CT sample (n = 75, mean age 62 years) did not answer the 4-month follow-up questionnaire. Of those lost to follow-up, three had their own personal reasons withdrawn from adjuvant CT, seven patients (3 < 65 years and 4 ≥ 65 years) withdrew during the six cycles due to various medical problems. The reasons were diverticulitis (n = 1), neutropenia (n = 2), and relapse (n = 2) pulmonary embolism (n = 1), and heart conditions (n = 1), and the remaining eight received all CT courses but did not return the surveys despite a letter of reminder.
Additionally, seven patients (2 < 65 years and 5 ≥ 65 years) had a dose reduction due to low platelet count (n = 2), and neutropenia (n = 3) to rash (n = 1), nausea (n = 1).
Measures
Sociodemographic characteristics (e.g. marital status, education, work status, co-morbidities, and medication) were obtained through patient interviews and clinical variables (e.g. histopathology, surgery, stage) by review of medical records.
The core questionnaires used were the cancer-specific EORTC QLQ-C30 Citation[15] and the tumour-specific breast cancer module EORTC QLQ-BR23 Citation[16]. The reliability of the Swedish version has been assessed in both healthy individuals and different groups of cancer patients, with internal consistency (Cronbach‘s alpha) ranging from 0.55 to 0.87 Citation[17].
The 14-item Hospital Anxiety and Depression Scale (HADS) was used to assess anxiety and depression Citation[18]. Cronbach's alpha for the Swedish version of HADS is 0.84 for anxiety and 0.82 for depression Citation[19].
Statistical analyses
For each patient and each HRQoL subscale a linear regression coefficient was calculated to describe the trend with time from baseline during treatment. Fisher's test for paired comparisons was used to test if the regression coefficients were significantly different from zero, i.e. if there were any trends with time from baseline during treatment. Differences between baseline and the mean of the first, third and the sixth cycle were calculated to elucidate the difference between baseline and treatment time. Differences between baseline and the 4-month follow-up were also calculated to elucidate the difference between baseline and follow-up. Correlations between age and the described differences were tested with Pitman's test Citation[20]. Pitman's test, Fisher's permutation test or Kruskal-Wallis test, which all are non-parametric tests, were used to test for relations between age and sociodemographic as well as clinical variables (which of these tests that were used depended on type of variable tested). Patients with missing values on a variable were excluded from analyses in relation to that variable. All tests were two-tailed and conducted at 5% significance level.
Note that all tests were performed with age as a continuous variable, but for descriptive purposes Tables and show data divided into a younger group (55–64 years; n = 36) and an older group (65+ years; n = 39). This age cut-off was chosen for two reasons: 1) Most clinical HRQoL studies of breast cancer have excluded those over 65 years; 2) it divides the total group approximately at the median. A separate analysis of drop-outs was performed to study whether these patients differed regarding age and baseline HRQoL.
Figure 1. Box plots of Global health status/QoL from the EORTC QLQ-C30. Boxes with solid lines are younger patients (age 55–64) and boxes with dashed lines are older patients (age 65–77). The boxes indicate the interquartile range, the 25th to the 75th percentile.
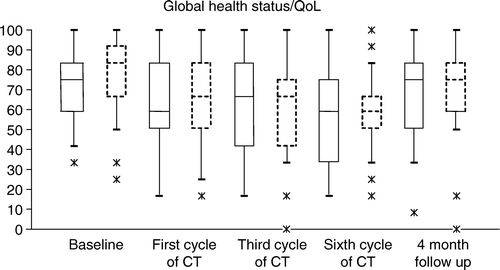
Table I. Sociodemographic and clinical characteristics of the sample, divided into younger patients (age 55–64) and older patients (age 65–77).
Table II. Mean values (SD) of the EORTC QLQ-C30 scales, divided into younger patients (age 55–64) and older patients (age 65–77).
Table III. Mean values (SD) of the EORTC BR-23 and HAD scales, divided into younger patients (age 55–64) and older patients (age 65–77).
Ethics
The Ethics Committees at Göteburg University and Stockholm University approved the study. All patients received oral and written information, and gave informed written consent before inclusion.
Results
Sociodemographic and clinical characteristics
Sociodemographic and clinical characteristics of the sample are presented in . As expected, age was significantly related to retirement (Fisher's permutation test; p < 0.001) and level of education (Fisher's permutation test; p < 0.05). However, there were no significant differences in marital status due to age. The only co-morbidity related to age was cardiovascular disorders, which were more common among older (Fisher's permutation test; p < 0.05). Older women also used significantly more medication (data not shown; Fisher's permutation test; p < 0.001), particularly cardiovascular medication (data not shown; Fisher's permutation test; p < 0.05). The 18 (24%) patients that were lost to 4-month follow-up did not differ statistically from the total sample at baseline, and there was no significant correlation between age and completion of the follow-up questionnaire. The mean age of these 18 patients was 65 years and the range was 55–77 years.
HRQoL and symptom experience depending on age and over time
There was a significant decrease in global health status, body image (BR-23), physical-, role-, social- (all; Pitman's test; p < 0.001) and cognitive functioning (Pitman's test; p < 0.05) during the CT period for all women ( and ). These decreases, except for social and cognitive functioning, had not fully recovered to baseline levels 4 months after the end of CT. There was no significant change in future perspective, emotional and sexual functioning from baseline through the treatment period and to time of follow-up. The level of anxiety decreased for all women during treatment (Pitman's test; p < 0.01) and was significantly lower at time of follow-up (Pitman's test; p < 0.001) compared with baseline ().
There were also significant increases in depression, upset by hair loss, systemic therapy side effects, nausea and vomiting, fatigue, dyspnoea, appetite loss (all; Pitman's test; p < 0.001), and pain (Pitman's test; p < 0.01) over the treatment period; whereas sleep, diarrhoea and financial difficulties did not change. These increased values had not fully recovered to baseline values at 4 months after the end of CT, except for nausea/ vomiting, appetite loss, constipation, and upset by hair loss. Sleep (Pitman's test; p < 0.01) and financial difficulties (Pitman's test; p < 0.001) were significantly worse at baseline. Breast and arm symptoms did not significantly change over the treatment period, but increased from baseline to time of follow-up (Pitman's test; p < 0.001 and p < 0.01, respectively).
There were no significant relationships between age and any of the HRQoL scales, expect for dyspnoea (Pitman's test; p < 0.001) and sexual functioning (Pitman's test; p < 0.01). Age was inversely related to worsening of dyspnoea over time, whereas perceived sexual functioning was lower at baseline for older women.
Discussion
Age did not seem to have an impact on changes in HRQoL during and after adjuvant CT in this study of 75 consecutively treated postmenopausal patients. The only variables significantly related with age were dyspnoea (worsening of symptoms was inversely related to age) and sexual functioning (older women had lower sexual functioning at baseline). Looking at HRQoL over time there was a significant decrease in global health status, body image, physical and role functioning during and after completed treatment, but the decrease was independent of age. These results indicate that concern about decrease in HRQoL would not alone be a reason not to consider CT among elderly.
However, the generalization of the results must be interpreted with caution since this is a hospital-based study with consecutively treated postmenopausal patients and we lack information about patients who were not referred for or not offered adjuvant CT, or who declined participation. Indeed, when we compared our study results with a healthy reference group from another study consisting of younger (40–49 years) and older women (70–79 years) Citation[17], our older patients had significantly better physical functioning at baseline than did the older referents. But, in a comparison with a reference group of younger (31–64 years) and older (65–80 years) breast cancer patients Citation[21], we found that our patients reported worse global health, role-, physical-, and emotional functioning (all p < 0.05) and more symptoms (e.g., nausea, fatigue, sleep and pain; all p < 0.05) during treatment. Comparison with reference groups from other studies must be interpreted with caution.
A surprising result was that age had no impact on declines in physical functioning during treatment. This may possibly reflect a difference in the health expectations of younger and older women, and discordance between their health expectations and current experience Citation[22]. Individuals with high expectations of their health may experience substantial HRQoL impacts from a modest clinical condition, whereas those with lower expectations (e.g. older patients) may experience less deterioration in the same circumstances.
We also found that older women experienced more cardiovascular disorders. This result is consistent with the findings of Yanick et al. Citation[12] that approximately 30% (n = 319) of those aged 65–69 years had one or more major co-morbidities. Co-morbidity and increased age are key considerations in offering systemic adjuvant treatment to older women, since they can minimize the potential benefit of any adjuvant therapy, and even increase the side effects of breast cancer treatment Citation[5], Citation[12], Citation[23].
At 4-month follow-up, physical functioning had still not recovered to baseline levels and several symptoms were still prevalent. One reason for that may be the fact that almost all women had a 5-week radiotherapy course starting 3–4 weeks after CT, leaving a treatment-free interval of only 7 weeks before the 4-month follow-up. It is therefore important that more long-term follow-up studies should be conducted in order to see how adjuvant treatment affects HRQoL and experienced symptoms in the longer perspective.
Although patients generally reported less fatigue at 4 months follow-up compared to the last CT cycle, younger women in particular still felt fatigued. Some studies suggest that fatigue gradually decreases after the last treatment, but it is often seen as a frequent complaint in disease-free cancer patients up to months and even years after curative cancer treatment has ended Citation[24], Citation[25]. In a recently published review by Ganz et al. Citation[26] on fatigue in two survival populations, breast cancer and Hodgkin‘s disease the conclusion was that cancer-related fatigue persists long after cancer treatments end, and is associated with more intensive treatments (combined CT and RT) in these cancers. Research results on the relationship between age and fatigue are not consistent. In some studies age, as in the present one, is not related to fatigue levels Citation[27], in others, older women experience more fatigue Citation[28], and in yet other studies, younger patients experience more fatigue Citation[29]. This inconsistency highlights the need for more research regarding the experience of fatigue in elderly cancer patients Citation[24]. Since there are few studies that have focused solely on elderly cancer patients, the opportunity for comparison with other similar studies is limited. It is therefore of great importance that more and larger comparative studies should be performed in this elderly population.
The strengths of the current study are the demographic and diagnostic homogeneity of the sample, the use of well-established self-report instruments, and the longitudinal design. This study does, however, have some limitations. Loss of patients to follow-up represents a common and serious threat to the internal validity of a study. The 24% of our patients who were lost to follow-up did not differ at baseline from those who continued, but they may have differed over the course of treatment, resulting in potential biases of the results. We found however no statistically significant correlation between age and questionnaire completion at follow-up, and those who were lost to follow-up were equally distributed in the younger and older age groups, as were those who withdrew during the 6-cycle treatment program due to medical problems. However, older women were more prevalent (n = 5) among those who had a dose reduction during treatment (n = 7).
We also investigated whether or not the mode of questionnaire administration (personal interview at home/at hospital versus telephone interview) correlated to responded HRQoL. No significant differences were found (data not shown) between the modalities. The interview method did not either affect response rate.
Conclusions
In this study of postmenopausal patients with breast cancer consecutively treated with adjuvant CT, age was found to be unrelated to decreases in HRQoL and to the patterns of symptoms. The generalization of the results must be interpreted with caution since this is a selected hospital-based series of patients. This small piece gives further support to the argument that age should not be used in isolation in decisions about adjuvant CT for breast cancer in elderly women. However, further studies on harms and benefits of adjuvant CT among elderly breast cancer patients are needed.
Acknowledgements
We are most grateful to all the women who took part in the study. We wish to thank Gunnar Ekeroth, statistician, and Anders Oden, professor in biostatistics, for providing help and guidance with the statistical analyses. This study was funded and supported by the King Gustav V Jubilee Clinic Cancer Research Foundation, Göteborg, and the Sahlgrenska Academy at Göteborg University, Institute of Health and Care Sciences, Göteborg, Sweden.
References
- Socialstyrelsen [The National Board of Health and Welfare]. Cancer incidensen i Sverige 2004 [Cancer Incidence in Sweden 2004]. Centrum för Epidemiologi. Officiell statistik i Sverige 2004. Stockholm.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Eaker S, Dickman PW, Bergqvist L, Holmberg L. Differences in management of older women influence breast cancer survival: Results from a population – based database in Sweden. PLoS Medicine 2006; 3: 1–8
- Whiterby SM, Muss HB. Special issues related to breast cancer adjuvant therapy in older women. Breast 2005; 14: 600–11
- Muss HB. Adjuvant therapy for older women with breast cancer. Breast 2003; 12: 550–7
- Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA 2005; 293: 1073–81
- Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer: Using a mediation model to test a symptom cluster. Oncol Nurs Forum 2005; 32: 542
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 2002; 24: 471–80
- Bender CM, Ergyn FS, Rosenzweig MQ, Cohen SM, Sereika SM. Symptom cluster in breast cancer across 3 phases of the disease. Cancer Nurs 2005; 28: 219–25
- Ganz PA, Kwan L, Stanton AL, Krupnick JC, Rowland JH, Meyerowitz BE, et al. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst 2004; 96: 376–87
- De Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs 2002; 25: 283–97
- Yanick R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and co-morbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001; 285: 885–92
- Green JM, Hacker ED. Chemotherapy in the geriatric population. Clin J Oncol Nurs 2004; 8: 591–7
- Aaronson NK, Cull AN, Kaasa S, Sprangers MAG. The European Organization for Research and Treatment of Cancer (EORTC) Modular approach to quality of life assessment in oncology. Quality of life and Pharmacoeconomics in Clinical Trials. An update. 2nd ed. Spilker B, Philadelphia: Lippincott-Raven Publishers; 1996. p 179–189.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30; a quality of life, instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76
- Sprangers MAG, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country study. J Clin Oncol 1996; 14: 2756–68
- Michelson H, Bolund C, Nilsson B, Brandberg Y. Health-related quality of life measured by the EORTC QLQ-C30- reference values from a larger sample of Swedish population. Acta Oncol 2000;39:477–84.
- Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scand 1983; 67: 361–70
- Lisspers J, Nygren A, Söderman E. Hospital anxiety and depression scale (HAD): Some psychometric data for a Swedish sample. Acta Psychiatrica Scand 1997; 96: 281–6
- Good P. Permutation tests. Springer Verlag, New York 2000
- Watters JM, Yau JC, O'Rourke K, Tomiak E, Gertler SZ. Functional status is well maintained in older women during adjuvant chemotherapy for breast cancer. Ann Oncol 2003; 14: 1744–50
- Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience?. BMJ 2001; 322: 1240–3
- Fehlauer F, Tribius S, Mehnert A, Rades D. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: Impact of age at therapy. Breast Cancer Res Treat 2005; 92: 217–22
- Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet 2003; 362: 640–50
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage 2004; 28: 373–80
- Ganz PA, Bower JE. Cancer related fatigue: A focus on breast cancer and Hodgkin‘s disease survivors. Acta Oncol 2007; 46: 474–9
- Geinitz H, Zimmermann FB, Thamm R, et al. Fatigue in patients with adjuvant radiation therapy for breast cancer: Long-term follow-up. J Cancer Res Clin Oncol 2004; 130: 327–33
- Respini D, Jacobsen PB, Thors C, Tralongo P, Balducci L. The prevalence and correlation of fatigue in older cancer patients. Crit Rev Oncol/Heamtol 2003; 47: 273–9
- Respini D, Tralongo P, DiMari A. Fatigue and depression in older cancer patients treated with chemotherapy. In: Proceedings of the paper presented at the UICC (International Cancer Congress) (Abstract 0112). Oslo: 2002.