Abstract
Purpose. To investigate the response rate of the triple combination of oxaliplatin (L-OHP) in combination with irinotecan (CPT-11) and 5-fluorouracil (5-FU) and to assess its impact on secondary resectability of previously non-resectable liver metastasis (LM). Patients and methods. Patients≥18 with MCRC, ECOG grade 0–2, and no prior treatment received L-OHP (85 mg/m2), CPT-11 (150 mg/m2) and 5-FU (2 250 mg/m2 in 48 h CI) on D1 every 15 days. Results. Forty-seven patients with initially non-resectable metastatic disease were included. Median age 62 years (38–76); 28 males; 26 patients with 0 performance status (ECOG) 40 patients had prior surgery and four adjuvant chemotherapy. All patients were evaluable for toxicity and 42 for response. Main grade 3–4 toxicities were neutropenia (40%), febrile neutropenia (4%), diarrhea (21%), nausea/vomiting (11%/15%), fatigue (11%), anemia and alopecia (9% each); grade 3–4 neurotoxicity was observed in 28% patients. Secondary surgery was possible in 15 of 47 (31.9%) patients and 12/30 (40%) patients with only LM: in this cohort, median OS has not been reached at 22 months median follow-up, with 2/12 patients having died. Overall response rate was 69% (95% CI, 53–82%); 13 (31%) had stable disease. Median time to progression and overall survival (OS) were 10.9 (95% CI, 9.9–13.2) and 19.9 (95% CI, 11.7-TBD) months, respectively. Conclusion. This combination has shown promising activity with manageable toxicity as front-line treatment in MCRC, and has allowed the resectability of LM in a considerable number of patients, offering them the possibility of long-term survival.
For several decades, the standard treatment for metastatic colorectal cancer (MCRC) has been 5-fluorouracil (5-FU)-based chemotherapy, despite having no major impact on survival Citation[1]. Recently, multiagent chemotherapy, consisting of either irinotecan (CPT-11) or oxaliplatin (L-OHP) combined with 5-fluorouracil (5-FU)±leucovorin (LV), has largely supplanted single-agent chemotherapy as first line treatment for MCRC. This change has been based on several phase III trials that have demonstrated that the addition of L-OHP or CPT-11 to 5-FU in first-line treatment significantly improves the results in these patients Citation[2–6]. Of interest is that those phases III studies have clearly shown that the advantage of an up-front, more aggressive approach is still evident even if active second-line therapies are offered to patients whose disease is progressing on 5-FU/LV. Moreover, the link between tumor response and first-line chemotherapy and survival has been supported by a meta-analysis Citation[7]. Furthermore, resection of metastatic disease after a highly active first-line chemotherapy regimen is possible in a small but important subset of patients with MCRC, particularly after receiving an oxaliplatin-based chemotherapy regimen, with encouraging overall survival and time to progression Citation[2], Citation[8]. In these patients, this is the only treatment option that currently offers a chance for long-term survival, in spite of being associated with a poor outcome in patients with multinodular MCRC. Due to its better efficacy, chemotherapy is increasingly proposed as neoadjuvant treatment in such patients to allow or to facilitate the radicality of resection. Indeed, Adam et al. observed that a good survival benefit from resection of liver metastases could only be obtained in patients responding to neoadjuvant chemotherapy, so a powerful neoadjuvant regimen is crucial in this kind of patient Citation[9].
Nevertheless, despite treatment improvement, a number of patients are non-responders to a double combination and also some of them cannot receive second-line treatment after progression to first-line chemotherapy. One recently reported review of seven phase III trials suggests that the use of all three active drugs (5-FU, L-OHP and CPT-11) in MCRC produces the longest overall survival Citation[10]. Therefore, the next step has been the development of strategies to integrate the three most active drugs in advanced colorectal cancer. The question is if these drugs will be more effective/better tolerated when used simultaneously or sequentially. Tournigand et al. Citation[11] randomized previously untreated patients to receive the simplified LVFU2 DeGramont regimen Citation[12] combined either with irinotecan (FOLFIRI) or with oxaliplatin (FOLFOX6): at progression, irinotecan was replaced by oxaliplatin (arm A), or oxaliplatin by irinotecan (arm B). A limitation of this approach is that the sequential treatment with all three agents cannot be guaranteed for all patients. Therefore, the use of these drugs simultaneously might be considered in order to increase the number of patients exposed to them before progressing. Triple-combination protocols using 5-FU plus CPT-11 plus L-OHP plus 5-FU±LV have consistently resulted in high response rates in patients with previously untreated metastatic CRC Citation[13–19] and recently have proved their superiority in a phase III trial which compared this with FOLFIRI Citation[20]. These studies have used several schedules, most of them based on a biweekly administration of 5-FU/LV or without LV. Previously, we conducted a phase I trial of L-OHP combined with CPT-11 and 48-hour continuous infusion (CI) of 5-FU alone Citation[21]. The maximum-tolerated dose was not reached, and thus the recommended doses were the same as when administered in combination therapy of L-OHP+5-FU or CPT-11 + 5-FU. In a previous trial we reported that the recommended doses of weekly 5-FU continuous infusion plus biweekly oxaliplatin are 2.25 g/m2 and 85 mg/m2 respectively Citation[22].
The purpose of this multicenter phase II study was to evaluate the antitumor activity and safety of this schedule, as first-line treatment in patients with MCRC, in order to increase secondary resectability in initially non-resectable or poor prognosis liver metastases.
Patients and methods
Eligibility criteria
Patients with histologically proven adenocarcinoma of the colon or rectum, with evaluable non initially operable metastatic disease, were enrolled. Criteria for initially non-resectable patients were: more of 4 nodes bilobar, node >10 cm, vascular involvement or extrahepatic disease. All patients had at least one of these criteria to be included in the study. Previous chemotherapy for metastatic disease was not permitted; previous adjuvant chemotherapy was allowed if finished at least 6 months before starting study treatment. Other eligibility criteria included the following: age ≥ 18, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; absolute neutrophil count ≥ 1.5×109/L and platelets ≥ 100×109/L; total bilirubin ≤ 1.5 times the upper normal limit (UNL) and serum transaminase levels ≤ 2.5 times UNL; creatinine clearance ≥ 50 ml/min; absence of a second primary tumor other than non-melanoma skin cancer or in situ cervical carcinoma. Patients with uncontrolled serious concomitant disease, intestinal obstruction, inflammatory enteropathy history, peripheral neuropathy or symptomatic brain/leptomeningeal metastasis were excluded. The protocol was designed following the recommendations of the Helsinki Declaration. The Ethics Committees of each institution approved the protocol, and signed informed consent was obtained from all patients.
Treatment schedule
Patients received L-OHP (85 mg/m2), CPT-11 (150 mg/m2) and 5-FU (2250 mg/m2 in 48 h CI-TTD scheme), the recommended doses in our previous phase I study Citation[21]. Drugs were delivered as follows: day 1, 2 h infusion of L-OHP followed by 30 min infusion of CPT-11 and 48 h infusion of 5-FU. Routine antiemetic prophylaxis with a 5-hydroxytryptamine-3-receptor antagonist was used. Subcutaneous atropine (0.25 mg) was recommended from the first cycle. Patients were given instructions for treating diarrhea by abundant fluid intake, loperamide 2 mg every 2 h for at least 12 h, and ciprofloxacin 500 mg/12 h in case of diarrhea lasting more than 24 hours. Treatment was administered every 2 weeks until evidence of progression, unacceptable toxicity or hepatic metastasis could be resected.
Patients were assessed for toxicity before each cycle using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC), version 2.0, April 1999. Chemotherapy was delayed if, on the planned day of treatment, neutrophils were less than 1.5×109/l or platelets less than 100×109/l or for significant persisting non-hematologic toxicity. Doses of all drugs were reduced in subsequent cycles (CPT-11 and 5-FU by 20% and L-OHP to 65 mg/m2) in case of grade 4 neutropenia, thrombocytopenia, diarrhea or stomatitis; if grade 3 neutropenia, thrombocytopenia, diarrhea or stomatitis appeared, only CPT-11 and 5-FU drugs were reduced; the appearance of grade 3–4 hand-foot syndrome was an indication for a 20% reduction in 5-FU alone; L-OHP dose was reduced to 65 mg/m2 in cases of temporary (7 to 14 days) painful paresthesia or functional impairment; if persistent (≥14 days) not painful paresthesia appeared, L-OHP was omitted in subsequent cycles from the regimen until recovery and then reinitiated at a reduced dose of 65 mg/m2; in cases of persistent (≥14 days) painful paresthesia or functional impairment, L-OHP was interrupted.
Evaluations during the study
During the 14 days before the first infusion, patients were interviewed and underwent physical examination, complete blood cell counts and differential, and blood chemistry (liver and kidney function tests). ECG, carcinoembryonic antigen (CEA) determination and tumor extension study (MRI or CT scan) were performed in the 4 weeks prior to treatment start. During treatment, blood cell counts were performed at the beginning of each cycle, and blood biochemistry every two cycles. CEA determinations and tumor response studies were performed using the WHO criteria every four 2-week cycles (8 weeks) or sooner if clinically indicated until the disease progressed or the patient died Citation[23]. Patients were resected during tumor response (or stabilization if 12 cycles of CHT were completed). After resection, patients were treated with postoperative CHT until a total of 6 months with pre and post CHT completed.
Statistical considerations
To determine the objective response activity of this regimen the minimax two-stage sequential design described by R. Simon Citation[24] was used to calculate the number of patients to be enrolled. Assuming a minimum efficacy (p0) of 40%, equivalent to that obtained with the standard reference combination of CPT-11 plus 5-FU/LV, a response rate of 60% or greater for a new regimen (p1) should be considered promising. With a level of significance of 95% (alpha error = 0.05) and a statistical power of 80% (beta error = 0.20), a sample size of 46 patients was required. The distribution of time to progression (TTP) and time to death (OS) were determined from the date of the beginning of the treatment using the Kaplan-Meier method, and the 95% confidence intervals were calculated using methods for exact binomial confidence intervals.
Results
A total of 47 patients were enrolled into the study. A summary of baseline patient characteristics is shown in . Mean age was 60.6 years (Standard Deviation 9.5) and all but one patient had an ECOG performance status of 0 to 1. CEA mean baseline value was 203.12 ng/ml (Standard Deviation 305.47).
Table I. Patients’ characteristics.
Treatment compliance
A total of 489 cycles were administered with a median per patient of 11 (range 1–18). Eighty seven cycles (17.8%) were delayed (33 patients, 70.2%). The delivered median relative dose-intensity for L-OHP, CPT-11 and 5-FU were 97%, 95% and 96% of the protocol-planned doses, respectively.
Toxicity
All patients were assessable for toxicity. The main grade 3–4 hematological and non-hematological toxicities are summarized in . Overall, 40% of patients experienced grade 3–4 neutropenia, with two cases of febrile neutropenia. The most frequent grade 3–4 non-hematological toxicities were diarrhea (21%), nausea/vomiting (11%/15%), fatigue (11%), anemia and alopecia (9% each). Neurotoxicity was observed in 13 (28%) patients, and reached grade 3–4 in two of them. No treatment related deaths were observed.
Table II. Grade 3–4 toxicity per patient and cycle according to NCI-CTC 2.0 criteria. * Febrile neutropenia: 2 (4.3%).
Response to treatment
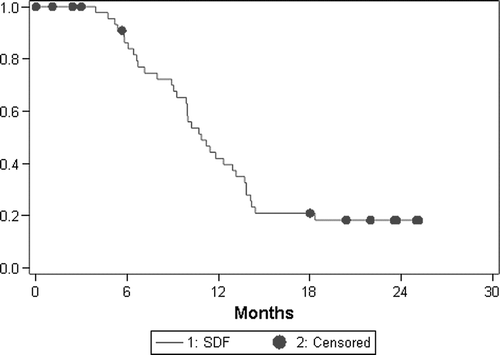
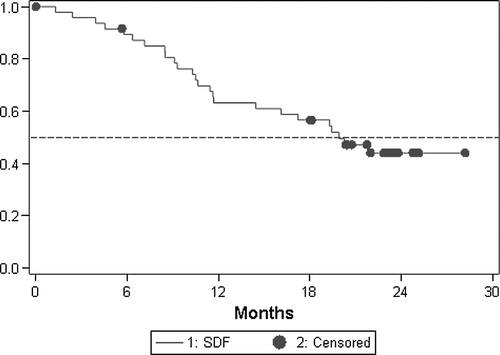
In an intention-to-treat analysis, an overall response rate of 61.7% (CI 46.4–75.5%) was documented. In the evaluable for response population, complete response was achieved in one patient for an overall response rate of 69% (CI 53–82%); 13 (31%) had stable disease (). Median time to progression and overall survival were 10.9 (CI 9.9–13.2) and 19.9 (CI 11.7–TBD) months, respectively ( and ).
Table III. Antitumoral efficacy. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, non evaluable.
Surgery after study chemotherapy
The median cycles of neoadjuvant chemotherapy for resected patients was six. Secondary surgery to remove residual disease was attempted in 15 patients (31.9%, CI 19.1–47.1), two patients thoracotomy, one patient hysterectomy and anexectomy and 12 patients liver resection. In the group of 30 patients with liver metastasis only, 12 of them (40%) were resected (95% CI, 22.7–59.4) with a R0 surgery of 66.6%. Two patients with liver relapse were treated with radiofrequency ablation (RFA). One patient with liver metastasis only achieved complete remision. This patient was not operated and did not have a relapse at the moment the study was closed. These 12 patients surgically resected, after a median follow-up of 22.31 months, median overall survival has not been reached, with 2/12 (17%) patients having died.
Discussion
Recently the results of a number of well-designed clinical trials conducted across the world have led to important advances in the management of MCRC. These iterative studies fostered the evolution from a standard single-agent approach using 5-FU to new combination regimens including irinotecan and oxaliplatin in addition to 5-FU. The integration of oxaliplatin and irinotecan for the management of patients with advanced disease has extended median survivals in a meaningful way Citation[2–6]. The identification of new combination chemotherapy regimens is an area of active clinical investigation. The potential synergism, no complete cross-resistance and different dose-limiting toxicities allow the combination of the three more active drugs in MCRC into a single regimen without significant dose reductions. In our schedule, we did not add leucovorin based on our previous experience in two consecutive phase II studies, which showed that the addition of LV to very high doses of 5-FU does not increase activity, but does toxicity Citation[25], Citation[26]. The dose of 3 500 mg/m2 5-FU is the standard adopted by the group based on a phase I study of a 48 h continuous 5-FU infusion Citation[27] and it is reduced to 2 250 mg/m2 in combination Citation[22], Citation[28]. Based on our previously reported phase I Citation[21] study, we have proposed a triple combination maintaining the same doses as when they are used combining only two of them.
In the present study the regimen was associated with high degree of antitumor activity that compared favourably with that so far reported for two-drug combinations which have been in the range of 40 to 63% Citation[2–6], Citation[11], Citation[22], Citation[28–33] and is in line with that obtained by other groups who have also tested regimens with these three drugs plus LV Citation[13–19]. However, an interesting finding was that all evaluable patients had disease control (CR + PR + SD). As has been mentioned above, Adam et al. Citation[9] found significantly worse five-year survival in patients who underwent hepatectomy during tumor progression after neoadjuvant chemotherapy, than in those with objective tumor response or disease stabilization, so tumor control before surgery is crucial to offer a chance of prolonged remission in patients with multiple metastases. The high level of activity attained has allowed us to perform hepatic resection in 40% of the patients with previously non-resectable liver metastases, superior to the 26–36% rate reported by Falcone et al. Citation[17], Citation[20], the 29% reported by Gil-Delgado et al. Citation[13], and the 37.5% communicated by Quenet Citation[34] all using a triple combination plus LV. Our results are similar to the 40.9% complete resection rate communicated by De La Camara in previously unresectable liver metastasis using FOLFORINOX regimen Citation[35], Citation[36]. Recently the NCCTG has reported a prospective clinical trial in patients with non-resectable liver metastasis Citation[37]. Patients were preoperatively treated with FOLFOX4 and revaluated before surgery. Sixty percent of patients had objective response and 17 (40%) were operated. In 14 patients a complete resection was performed and 67% of them were alive after 3 years of follow-up. Despite that 10% patient might have been candidate to initial surgery, as the authors and other suggest Citation[38], there is a clear relationship between response and resectability in this prospective clinical trial. In our trial, focused in non-initially resectable patients, non-resectability criteria were clearly established at study entry and all patients presented one or more criteria. Our preliminary data on survival are very promising in the liver metastasis only sub-population. In this group, after a median follow-up of almost 2 years, median overall survival has not been reached, with only two of 12 resected patients having died. Also, the median time to progression and survival reached in the global population is higher than the previously one reported in the literature up to date.
The good tolerability of this regimen allowed the effective administration of the planned dose intensity of the three drugs throughout the whole treatment period. As expected, the most frequent adverse events were consistent with those reported in patients treated with two of the three drugs used in this study: neutropenia, diarrhea and nausea/vomiting. Even taking into account that a comparison with previous phase II studies can be only speculative, this regimen seems to be better tolerated that others using a similar schedule, mainly with reference to hematological toxicity Citation[17], Citation[18]. Although 40% of patients experienced grade 3–4 neutropenia only in two cases this was complicated with febrile neutropenia. Neurotoxicity was observed in 25% of patients, but only graded 3–4 in two of them. No treatment related deaths occurred.
In conclusion, the results of this trial confirm those reported in our previous phase I study, showing that this biweekly combination of L-OHP, CPT-11 and 5-FU without leucovorin is feasible, with promising antitumor activity, given the possibility of resection of metastases offered to a considerable proportion of initially unresectable patients and, thus, improving their possibility to reach longer survival.
References
- Bertino JR. Chemotherapy of colorectal cancer: History and new themes. Semin Oncol 1997; 24: S18–3–S18–7
- Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000; 18: 136–47
- De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
- Goldberg RM, Morton RF, Sargent DJ, et al. N9741: Oxaliplatin or CPT-11 plus 5-fluorouracil/leucovorin or oxaliplatin plus CPT-11 in advanced colorectal cancer. Updated efficacy and quality of life data from an intergroup study. Proc Am Soc Clin Oncol 2003;22:252 (abstr 1009).
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first line treatment for metastatic colorectal cancer: A multicenter randomized trial. Lancet 2000; 355: 1041–7
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000; 343: 905–14
- Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P. Relation between tumor response to first-line chemotherapy and survival in advanced colorectal cancer: A meta-analysis. Lancet 2000; 356: 373–8
- Delaunoit T, Alberts SR, Sargent DJ, et al. Chemotherapy permits resection of metastatic colorectal cancer: Experience from Intergroup N9741. Ann Oncol 2005; 16: 425–9
- Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052–61. Discussion 1061–4.
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004; 22: 1209–14
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 2004; 22: 229–37
- De Gramont A, Louvet C, André T, et al. A simplified bimonthly regimen with leucovorin and 5-fluorouracil for metastatic colorectal cancer. Proc Am Soc Clin Oncol 1997;16:287a (abstr 1019).
- Gil-Delgado MA, Bastian G, Guinet F, et al. Oxaliplatin plus irinotecan and FU-FOL combination and pharmacokinetic analysis in advanced colorectal cancer patients. Am J Clin Oncol 2004; 27: 294–8
- Goetz MP, Erlichman C, Windebank AJ, et al. Phase I and pharmacokinetic study of two different schedules of oxaliplatin, irinotecan, fluorouracil, and leucovorin in patients with solid tumors. J Clin Oncol 2003; 21: 3761–9
- Ychou M, Conroy T, Seitz JF, et al. An open phase I study assessing the feasibility of the triple combination: Oxaliplatin plus irinotecan plus leucovorin/ 5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol 2003; 14: 481–9
- Comella P, Casaretti R, De Rosa V, et al. Oxaliplatin plus irinotecan and leucovorin-modulated 5-fluorouracil triplet regimen every other week: A dose-finding study in patients with advanced gastrointestinal malignancies. Ann Oncol 2002; 13: 1874–81
- Falcone A, Masi G, Allegrini G, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: A pilot study in patients with metastatic colorectal cancer. J Clin Oncol 2002; 20: 4006–14
- Souglakos J, Mavroudis D, Kakolyris S, et al. Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: A multicenter phase II trial. J Clin Oncol 2002; 20: 2651–7
- Calvo E, Cortes J, Gonzalez-Cao M, et al. Combined irinotecan, oxaliplatin and 5-fluorouracil in patients with advanced colorectal cancer: A feasibility pilot study. Oncology 2002; 63: 254–65
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) compared with Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25: 1670–6
- Abad A, Massuti B, Gallego J, et al. Phase I study of the combination of oxaliplatin, irinotecan and continuous infusion 5-fluorouracil in digestive tumors. Anticancer Drugs 2004; 15: 469–71
- Abad A, Carrato A, Navarro M, et al. Two consecutive phase II trials of biweekly oxaliplatin plus weekly 48-hour continuous infusion of nonmodulated high dose 5-fluorouracil as first line treatment for advanced colorectal cancer. Clin Colorectal Cancer 2005; 4: 384–9
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1
- Díaz-Rubio E, Aranda E, Camps C, et al. A phase II study of weekly 48-hour infusions with high-dose fluorouracil in advanced colorectal cancer: An alternative to biochemical modulation. J Infus Chemother 1994; 4: 58–61
- Aranda E, Cervantes A, Dorta J, et al. A phase II trial of weekly high-dose continuous infusion 5-fluorouracil plus oral leucovorin in patients with advanced colorectal cancer. Cancer 1995; 76: 559–63
- Díaz-Rubio E, Aranda E, Martín M, Gonzalez-Mancha R, Gonzalez-Larriba J, Barneto I. Weekly high-dose infusion of 5-fluorouracil in advanced colorectal cancer. Eur J Cancer 1990; 26: 727–9
- Aranda E, Carrato A, Cervantes A, et al. Phase I/II trial of irinotecan plus high-dose 5-fluorouracil (TTD regimen) as first-line chemotherapy in advanced colorectal cancer. Ann Oncol 2004; 15: 559–67
- Wasserman E, Cuvier C, Lokiec F, et al. Combination of oxaliplatin plus irinotecan in patients with gastrointestinal tumors: Results of two independent phase I studies with pharmacokinetics. J Clin Oncol 1999; 17: 1751–9
- Scheithauer W, Kornek GV, Raderer M, et al. Combined irinotecan and oxaliplatin plus granulocyte colony-stimulating factor in patients with advanced fluoropyrimidine/leucovorin-pretreated colorectal cancer. J Clin Oncol 1999; 17: 902–6
- Goldwasser F, Gross-Goupil M, Tigaud JM, et al. Dose escalation of CPT-11 in combination with oxaliplatin using an every two weeks schedule: A phase I study in advanced gastrointestinal cancer patients. Ann Oncol 2000; 11: 1463–70
- Scheithauer W, Kornek GV, Raderer M, et al. Randomized multicenter phase II trial of oxaliplatin plus irinotecan versus raltitrexed as first-line treatment in advanced colorectal cancer. J Clin Oncol 2002; 20: 165–72
- Scheithauer W, Kornek GV, Ulrich-Pur H, et al. Oxaliplatin plus raltitrexed in patients with advanced colorectal carcinoma. Cancer 2001; 91: 1264–71
- Quenet F, Nordlinger B, Riovire M, et al. Resection of previously unresectable liver metastases from colorectal cancer (LMCRC) after chemotherapy (CT) with CPT-11/L-OHP/LV5FU(Folfirinox): A prospective phase II trial. Proc Am Soc Clin Oncol 2004;23:273 (abstract 3613).
- De La Camara J, Rodriguez J, Rotellar F, et al. Triplet therapy with oxaliplatin, irinotecan, 5-fluorouracil and folinic acid within a combined modality approach in patients with liver metastases from colorectal cancer. Proc Am Soc Clin Oncol 2004;23:268 (abstract 3591).
- Leonard G, Brenner B, Kemeny N. Neoadjuvant chemotherapy before live resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2005; 23: 2038–48
- Alberts SR, Horvarth WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group Phase II study. J Clin Oncol 2005; 23: 9243–9
- Chong G, Cunningham D. Improving long-term outcomes for patients with liver metastases from colorectal cancer. J Clin Oncol 2005; 23: 9063–90