Abstract
β-mannosidase, encoded by MANBA, has been suggested to be implicated in cancers, while genetic variations in the MANBA in relation to colorectal cancer (CRC) risk has not been examined. In this study, we investigated the relationship of a polymorphic CA repeat in MANBA gene with CRC risk in 152 Swedish CRC patients and 441 Swedish controls, and 196 Chinese CRC patients and 577 Chinese controls, as well as the clinicopathologic significance of this polymorphism on CRC patients, by using capillary electrophoresis. The MANBA genotypes were related to CRC risk in the Swedish population (p=0.03), but not in the Chinese population. In the Swedish population, individuals with < 22 CAs/< 22 CAs had significantly increased risk for CRC compared with those with ≥22 CAs/≥ 22 CAs (gender-age-adjusted analysis: OR 1.93, 95% CI 1.06–3.51). There was no relationship between the polymorphism and clinicopathologic variables. These findings suggest the different susceptibilities of this polymorphism to CRC development in the two populations.
β-mannosidase is an exoglycosidase involved in the degradation of N-linked oligosaccharide moieties of glycoproteins. The gene, MANBA, encoding for β-mannosidase, comprises 17 exons and is located on chromosome 4q22-q25. It has been known that genetic deficiency of this enzyme results in beta-mannosidosis, a lysosomal storage disease that has a wide spectrum of neurological involvement Citation[1]. Recently, several studies have demonstrated the implication of β-mannosidase in cancers. Bosmann et al. Citation[2] reported elevated serum level of β-mannosidase in rats bearing transplantable Reuber H-35 hepatoma and mice bearing L1210 murine leukemia, suggesting that the tumor may release large quantities of β-mannosidase into the serum and affect the enzyme level in host organs. Sud et al. Citation[3] observed the overexpression of β-mannosidase in human esophageal dysplasia and squamous cell carcinoma compared with normal esophageal epithelium, indicating that β-mannosidase might be involved in human esophageal cancer development. However, the potential role of β-mannosidase and the genetic mechanism in tumor development and progression have not been reported.
Ota et al. Citation[4] first discovered a polymorphic dinucleotide CA repeat, thought in the regulatory region of NFKB1. They also observed that this polymorphism had a high heterozygosity (0.813), suggesting that this polymorphism is a useful marker for studying genetic disorders affecting immune response and cell differentiation. Studies have investigated the implication of the polymorphic CA repeat in several immune-related diseases including type 1 diabetes, multiple sclerosis and celiac disease, as well as breast cancer Citation[5–8]. Hegazy et al. found a significantly increased frequency of the allele 138 bp (24 CAs) and a decreased rate of the allele 146 bp (28 CAs) of the polymorphism in the patients with type 1 diabetes compared with the normal controls, suggesting the involvement of this polymorphism in the susceptibility to type 1 diabetes Citation[5]. However, this polymorphism did not associate with the susceptibility to multiple sclerosis, celiac disease and breast cancer Citation[6–8]. Recently, this polymorphism was identified in the MANBA gene (GenBank accession no. AF224669), not in NFKB1, approximately 300 bp downstream to exon 17 of the MANBA. In the present study, we investigated whether this polymorphism was related to colorectal cancer (CRC) risk in two geographically and ethnically different populations: Swedish and Chinese populations, and the clinicopathologic significance of the polymorphism on CRC patients.
Materials and methods
Subjects
This study included 152 Swedish consecutive CRC patients and 441 Swedish healthy controls, and 196 Chinese consecutive CRC patients and 577 Chinese healthy controls. The baseline characteristics of the patients and controls are shown in . Swedish patients consisted of 80 males and 72 females, aged 35–95 years (mean age, 71 years), diagnosed at Linköping Hospital, Linköping, or Vrinnevi Hospital, Norrköping, Sweden, between 1990 and 1999. The patients’ gender, age, tumor location and stage (Dukes’ and TNM stage) were taken from surgical and/or pathological records. Tumors from ascending and transverse colon were regarded as proximal tumors, while tumors from descending, sigmoid colon and rectum were considered as distal tumors. Tumor differentiation was graded as high (well + moderately) and low (poorly + mucinous) differentiated. No information was available about tumor location in two patients, Dukes’ stage in eight, TNM stage in 108, and differentiation in one. The patients were followed up until 2003, and 51 died of CRC by that time. Chinese patients consisted of 112 males and 84 females, aged 25–84 years (mean age, 57 years), diagnosed at the Department of Surgery, the Fourth Hospital, Hebei Medical University, Shijiazhuang, P. R. China, between 2001 and 2002, or Tangshan Worker's Hospital, Tangshan, P. R. China, between 2004 and 2005. The patients’ gender, age, tumor location, Dukes’ stage and differentiation were recorded by surgeons and pathologists, and confirmed by two of the present investigators. No information was available about age in one, tumor location in 40, Dukes’ stage in 23, differentiation in 107, and TNM stage and survival status in all patients.
Table I. The baseline characteristics of patients and controls in the Swedish and Chinese populations
Both Swedish and Chinese controls were collected from the same residential area as their corresponding patients. They had neither any gastrointestinal disease nor a personal history of tumors. Swedish controls consisted of 218 males and 223 females, aged 22–77 years (mean age, 55 years) recruited from a regional population-based DNA bank created in the south-east region of Sweden. Chinese controls were 370 male and 206 female blood donors, aged 18–88 years (mean age, 37 years) collected from Blood Research Center of Hebei Province, Shijiazhuang, P. R. China. No information was available about gender in one Chinese control, and age in one Swedish control and one Chinese control, respectively. The study was approved by the ethical committee at the Faculty of Health Sciences, University of Linköping, Sweden, and the Health Bureau of Hebei Province, Shijiazhuang, P. R. China.
DNA extraction
DNA from peripheral blood of the Swedish and Chinese controls, and the Chinese patients (who were from the Department of Surgery, the Fourth Hospital, Hebei Medical University, Shijiazhuang, China) was extracted by using the Wizard® Genome Purification Kit (Promega, Madison, WI). DNA from frozen normal colorectal mucosa of the Swedish patients, and Chinese patients (who were from Tangshan Worker's Hospital, Tangshan, China) was extracted by using Wizard® SV Genomic DNA Purification System according to the manufacturer's instructions (Promega).
Genotyping
Genotyping was examined by capillary electrophoresis with MegaBACE Fragment Profiler (Amersham Biosciences, Buckinghamshire, England) using the primers (forward primer FAM-labeled 5’-CTTCAGTATCTAAGAGTATCCT-3’ and reverse primer 5’-CAAGTAAGACTCTACGGAGTC-3’) described previously by Curran et al. Citation[8].
Polymerase chain reaction (PCR) was performed in a 20 µl reaction mixture containing approximately 50 ng genomic DNA, 0.5 µM of each primer (Invitrogen, Carlsbad, CA), 1× PCR buffer (Promega), 0.25 mM dNTPs (Invitrogen), 3.75 mM MgCl2 (Promega) and 0.025 U/µl Taq polymerase (Promega) at 94oC for 5 min, 35 cycles at 94oC for 30 s, 58oC for 30 s and 72oC for 30 s, and a finial extension at 72oC for 5 min. Following PCR amplification, 1 µl of the PCR product was subjected to 25 µl distilled water with 0.25 µl of MegaBACE ET 400-R size standard (Amersham Biosciences) and the mix was injected to the capillary system of the MegaBACE instrument. Consequently, the polymorphic alleles of MANBA were analyzed by MegaBACE fragment profiler.
DNA sequencing
In order to confirm the number of CA repeats in MANBA, genomic DNA from two Swedish and two Chinese patients with homozygous alleles 18 CAs or 23 CAs was sequenced by a MegaBACE 1000 sequencing instrument. Briefly, following DNA amplification by PCR with the same primers (neither fluorescence-labeled) and PCR conditions above, 5 µl of the PCR product was cleaned up with 2 µl ExoSAP-IT (Amersham Biosciences) by incubation at 37oC for 15 min first and then followed by inactivation through heating to 80oC for 15 min. The PCR product was then subjected to a sequencing reaction in a volume of 20 µl reaction mixture containing 7 µl PCR product, 1 µl reverse primer, and 8 µl sequencing reagent premix (Amersham Biosciences). A 25 cycles of the cycling program at 95oC for 20 s, 58oC for 15 s and 60oC for 1 min was performed. After purification, the sequencing reaction mixtures were detected by capillary electrophoresis by using the MegaBACE 1000 sequencing instrument and analyzed by MegaBACE 1000 sequencing profiler (Amersham Biosciences).
Statistical analysis
Power estimates (at a significant level of 5%) for the case-control analysis were calculated by one of the co-authors (Rosell, J, statistician) using the formulae described by Kirkwood BR Citation[9]. The student t-test was used to assess the age difference between patients and controls. The χ2 test was used to determine difference in the frequency of genotypes or alleles between patients and controls, between the two populations, as well as the relationship of the polymorphism with clinicopathologic variables. The logistic regression was used to calculate odds ratio (OR) and 95% confidence intervals (CI) in both univariate and multivariate analyses including gender and age for assessing the association of genotypes with CRC risk. Cox's Proportional Hazard Model was used to test the relationship between the polymorphism and the survival of patients. Kaplan-Meier method was used to calculate survival curves. Two-sided p-values of less than 5% were considered statistically significant.
Results
Power analysis
Under the case-to-control ratio of 1 to 2.9 (i.e., 152 Swedish patients versus 441 controls and 196 Chinese patients versus 577 controls), and the assumption of a prevalence of “bad” genotype of 17% in the Swedish control and 21% in the Chinese control, and a true odds ratio for cancer of 2, the sample size was associated with a power of about 90% for the Swedish and 95% for the Chinese study.
The distribution of MANBA alleles in the Swedish and Chinese populations
The age distribution between the patients and controls in both the Swedish and Chinese populations was significant difference (p < 0.0001, ). There was no gender difference between the patients and controls in both the populations (p > 0.05, ).
Thirteen polymorphic alleles, ranging from 116 to 144 bp corresponding to 13–27 CA repeats, were detected in the Swedish population (patients, 16–26 CAs, and controls, 13–27 CAs, A, ), and 15 alleles, ranging from 116 to 146 bp corresponding to 13 to 28 CA repeats, in the Chinese population (patients, 13–27 CAs, and controls, 14–28 CAs, B, ). The distribution of the alleles in both the populations was bimodal, with two peaks located at 18 CAs and at 24 CAs. However, the most common allele between the two populations was different: in the Swedish population, 24 CAs were the most common allele followed by 18 CAs, whereas in the Chinese population 18 CAs were the most common allele followed by 24 CAs. The distribution of the alleles between the two populations, either patients versus patients (χ2=59.37, γ = 12, p < 0.0001), or controls versus controls (χ2=83.56, γ = 14, p < 0.0001), was significantly different (). There was no difference in the frequency of alleles between the patients and controls either in the Swedish (χ2=18.77, γ = 12, p = 0.09) or in the Chinese populations (χ2=22.51, γ = 14, p = 0.07, data not shown). In addition, there was no gender-polymorphism association in the two populations (p > 0.05, data not shown).
Figure 1. Frequency distribution (%) of number of CA repeats in patients and controls in the Swedish (A) and Chinese (B) populations.
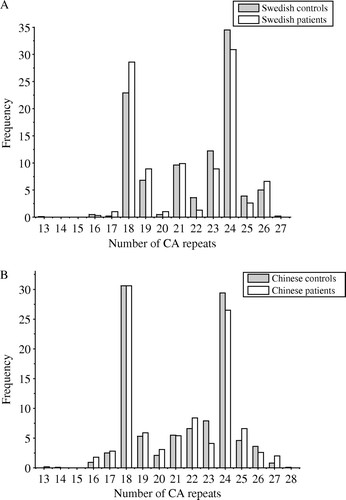
Table II. Frequency of MANBA alleles in patients and controls in the Swedish and Chinese populations
The correlation between the MANBA genotypes and CRC risk
Comparing the frequency of the same allele in the Swedish patients with the corresponding controls (e.g., at 13 CAs, 0 versus 0.1%, A), we found that almost all the alleles with < 22 CAs (except 13 CAs and 16 CAs) appeared more frequently in the patients than the controls (e.g., the frequency of allele with 21 CAs was higher in the patients (9.9%) than that in the controls (9.6%)), and an opposite trend was seen in alleles with ≥ 22 CAs (except 26 CAs) (e.g., the frequency of allele with 22 CAs was lower in the patients (1.3%) than that in the controls (3.6%)). Considering this trend of the allelic frequency difference between the Swedish patients and controls, together with the bimodal distribution of the CA repeats, a model adapted from previous analyses of dinucleotide microsatellite polymorphisms Citation[10–12], we divided the alleles into two subclasses using 22 CAs as a cut-off point to consequently obtain three groups of genotype,<22 CAs/ < 2 2 CAs,<22 CAs/ ≥ 22 CAs and ≥ 22 CAs/ ≥ 22 CAs, for further analysis. In the Swedish population, the genotype of the MANBA was significantly related to CRC risk with a dosage effect of allele with < 22 CAs (trend test in gender-age-adjusted analysis: p = 0.03, ). Further analysis showed that individuals with < 22 CAs/ < 22 CAs had a significantly increased risk for CRC compared with those with ≥ 22 CAs/ ≥ 22 CAs (OR 2.07, 95% CI 1.22–3.50, p = 0.01 for univariate analysis). Since the mean age of the Swedish patients differed from that of the controls (p < 0.0001, ), we further did a multivariate analysis adjusting for gender and age, and observed the similar results (OR 1.93, 95% CI 1.06–3.51, p = 0.03, ).
Table III. Association of MANBA genotypes with colorectal cancer risk in the Swedish and Chinese populations
There was no association of the MANBA genotypes with gender, age, tumor location, Dukes’ stage and TNM stage, grade of differentiation, and survival of the Swedish patients (p > 0.05, data not shown).
In the Chinese population, using the same cut-off point and subsequent genotype classification as above, we did not see any difference in the genotype frequency between the patient and controls (p > 0.05 for both univariate and multivariate analyses, ), or association of the genotypes with gender, age, tumor location, Dukes’ stage, and grade of differentiation of the Chinese patients (p > 0.05, data not shown).
Discussion
In the present study, the allele size and its corresponding CA repeat number (e.g., 126 bp was corresponding to 18 CAs, 138 bp was corresponding to 24 CAs) detected were consistent with the previous studies in MANBA (rather than in NFKB1) in a Japanese and a Spanish population Citation[4], Citation[7]. The distribution of the alleles in both the Swedish and Chinese populations was bimodal, with two peaks located at 18 CAs and at 24 CAs. However, the most common alleles between the two populations were different. In the Swedish population, the most common alleles were 24 CAs followed by 18 CAs, which were consistent with previous studies in Spanish population Citation[7]. However, in the Chinese population, the most frequent alleles were 18 CAs followed by 24 CAs. These results indicate that the distribution of the polymorphism among different ethnic populations may be different.
To our knowledge, the present study is the first investigation on the association of the polymorphic CA repeats in MANBA with CRC risk. A previous study has investigated this polymorphism for involvement breast cancer in the Australian population Citation[8]. In accordance with their finding, where the polymorphisms of the alleles (they did not analyze genotypes) were not related to risk for breast cancers Citation[8], we did not either find an association of the MANBA alleles with risk for CRC in both the Swedish and Chinese populations. Interestingly, when we used 22 CAs as a cut-off point to set three genotypes,<22 CAs/ < 22 CAs,<22 CAs/ ≥ 22 CAs and ≥ 22 CAs/ ≥ 22 CAs, genotype < 22 CAs/ < 22 CAs was related to CRC risk compared with ≥ 22 CAs/ ≥ 22 CAs in the Swedish population. These results indicated that not only the polymorphisms but also the CA repeat number played a role in the susceptibility to CRC development.
It is notable that this polymorphism was related to CRC risk in the Swedish but not in the Chinese population. Swedish and Chinese populations are two geographically and ethnically different populations with marked differences in CRC incidence rate, culture and life style. For example, the age-adjusted incidence rate of CRC among Swedish population (33.4/100 000 in males and 26.2/100 000 in females) is considerably higher than incidence among Chinese population (13.6/100 000 in males and 9.3/100 000 in females) Citation[13]. Swedish people have a higher red meat consumption and lower intake of plant foods while Chinese have opposite. Studies of migrants moving from a low- to a high-risk area have suggested that the variation in CRC incidence among populations is mainly due to difference of environmental factors and life style, most likely diet Citation[14], Citation[15]. Furthermore, accumulating evidence has shown that the interaction of environmental exposures with genetic polymorphisms may influence the susceptibility of polymorphisms to cancer Citation[16]. In the present study, we observed the different pattern of the allelic distribution between the Swedish and Chinese population, and statistical analyses revealed a significant difference of the frequency of MANBA alleles between the two populations. Taken together, the genetic difference attributable to different populations as well as environmental exposures may partly explain the discrepancy of the MANBA polymorphism in susceptibility to CRC as well as CRC incidence diversity between the two populations. On the other hand, there was an obvious age-difference between the patients and controls in the two populations, which may influence the evaluation of the effect of MANBA polymorphism on the susceptibility to CRC, although age-adjusted analysis has been done. In addition, polymorphisms in other genes or loci within the MANBA gene that were in linkage disequilibrium with the CA repeat polymorphism may also have impact on the susceptibility of the polymorphism to CRC in the two populations.
In conclusion, the association of the CA repeat polymorphism of MANBA gene with CRC risk in the Swedish but not in Chinese population suggests the different susceptibilities of this polymorphism to CRC development in the two populations. Further analysis containing strictly selective subject with matched age, genetic linkage, gene expression and environmental exposure in different populations would contribute to our understanding the function of the MANBA polymorphism.
Acknowledgements
The authors are grateful to Ms. Annette Molbaek for technique assistance of MegaBACE genotyping analysis. The study was supported by grants from the Swedish Cancer Foundation, and the Health Research Council in the south-east of Sweden.
References
- Alkhayat AH, Kraemer SA, Leipprandt JR, Macek M, Kleijer WJ, Friderici KH. Human beta-mannosidase cDNA characterization and first identification of a mutation associated with human beta-mannosidosis. Hum Mol Genet 1998; 7: 75–83
- Bosmann HB, Spataro AC, Myers MW. Serum and host liver activities of glycosidases and sialyltransferases in animals bearing transplantable tumors. Res Commun Chem Pathol Pharmacol 1975; 12: 499–512
- Sud N, Sharma R, Ray R, Chattopadhyay T, Ralhan R. Differential expression of beta mannosidase in human esophageal cancer. Int J Cancer 2004; 112: 905–7
- Ota N, Nakajima T, Shirai Y, Emi M. Isolation and radiation hybrid mapping of a highly polymorphic CA repeat sequence at the human nuclear factor kappa-beta subunit 1 (NFKB1) locus. J Hum Genet 1999; 44: 29–30
- Hegazy DM, O'Reilly DA, Yang BM, Hodgkinson AD, Millward BA, Demaine AG. NFkappaB polymorphisms and susceptibility to type 1 diabetes. Genes Immun 2001; 2: 304–8
- Miterski B, Bohringer S, Klein W, Sindern E, Haupts M, Schimrigk S, et al. Inhibitors in the NFkappaB cascade comprise prime candidate genes predisposing to multiple sclerosis, especially in selected combinations. Genes Immun 2002; 3: 211–9
- Rueda B, Lopez-Nevot MA, Ruiz MP, Ortega E, Maldonado J, Lopez M, et al. CA microsatellite polymorphism of the nuclear factor kappa B1 gene in celiac disease. Eur J Immunogenet 2004; 31: 129–32
- Curran JE, Weinstein SR, Griffiths LR. Polymorphic variants of NFKB1 and its inhibitory protein NFKBIA, and their involvement in sporadic breast cancer. Cancer Lett 2002; 188: 103–7
- Kirkwood BR. Calculation of required sample size. Essential of medical statistics 1988;191–200.
- McGinnis RE, Spielman RS. Insulin gene 5′ flanking polymorphism. Length of class 1 alleles in number of repeat units. Diabetes 1995; 44: 1296–302
- Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 2000; 66: 187–95
- Lee EY, Yim JJ, Lee HS, Lee YJ, Lee EB, Song YW. Dinucleotide repeat polymorphism in intron II of human Toll-like receptor 2 gene and susceptibility to rheumatoid arthritis. Int J Immunogenet 2006; 33: 211–5
- Ferlay J, Bray F, Pisani P, Parkin DM. Cancer incidence, mortality and prevalence worldwide, GLOB CAN 2002. Database, IARC, Lyon, 2002; www-dep.iarc.fr/GLOBOCAN.
- Bingham S, Riboli E. Diet and cancer – the European prospective investigation into cancer and nutrition. Nat Rev Cancer 2004; 4: 206–15
- Flood DM, Weiss NS, Cook LS, Emerson JC, Schwartz SM, Potter JD. Colorectal cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control 2000; 11: 403–11
- Mucci LA, Wedren S, Tamimi RM, Trichopoulos D, Adami HO. The role of gene-environment interaction in the etiology of human cancer: Examples from cancers of the large bowel, lung and breast. J Intern Med 2001; 249: 477–93