Abstract
Purpose. Neck dissection (ND) is routinely performed for persistent nodal disease after definitive chemo-radiotherapy (CRT) for locally advanced head and neck cancer. This study analyzes the role and extent of ND necessary after CRT based on pathologic outcome. Patients and methods. The study is based on 42 patients undergoing 48 ND's for persistent nodal disease after CRT. Patients were treated to a median radiation dose of 70.4 Gy at 1.8–2 Gy per fraction concurrently with platinum based chemotherapy. Patients with documented residual disease in neck, based on clinical or radiological examination underwent ND at a median interval of 59 days after completion of CRT. Results. Of the 42 patients undergoing ND, 11 (26%) had positive findings on pathologic evaluation. The clinical and treatment characteristics were similar for node negative and positive patients. The involved nodal level(s) were always confined within the clinically documented persistent disease. The median percentage of positive nodes to total nodes removed was 10%. Almost 50% of positive nodes removed had only microscopic or minute viable cancer pathologically. The outcome was better for pathologically node negative patients in comparison to node positive patients. Conclusion. The results of this study suggest that standard ND appears to be an excessive treatment for persistent nodal disease after CRT. Limited ND or even gross nodal resection confined to involved nodal level(s) as identified clinically or radiologically should be tested in a prospective randomized trial for reducing treatment related morbidity while maintaining excellent loco-regional control.
CRT has emerged as a standard of care for locally advanced head and neck cancer with resultant advantage of organ preservation Citation[1], Citation[2], surgery being reserved for salvage. However, modified/selective neck dissection (ND) is routinely performed after completion of concurrent CRT for persistent neck disease when primary is clinically and pathologically controlled. Due to lack of randomized data, the decision to perform ND or observe patients after CRT is controversial.
Several questions remain to be answered to standardize the indications of ND based on post-CRT clinical and radiological information. Should initial tumor burden dictate the planned ND or should this be based on response to therapy? The response rates after completion of CRT can be defined clinically, with conventional radiological studies such as CT, MRI or more recently with PET scans. The judicious use of this information to appropriately select patients for ND is lacking. There is no consensus on type of ND either which is arbitrarily surgeon or institution dependent. The current literature gives conflicting answers to the above-mentioned questions because of lack of randomized data Citation[3–15].
We routinely perform ND for patients who have proven persistent disease after CRT either clinically or radiologically. We present analysis of patients who underwent ND and correlate with pathologic outcome to provide information as to necessity and extent of surgery.
Patients and methods
Between 1999 and 2004, 48 NDs were performed in 42 patients for residual disease after concurrent CRT for the treatment of advanced squamous cell carcinoma (SCC) of the head and neck. The research protocol was approved by the Institutional Review Board. The patients were all 2002 American Joint Committee on Cancer Stages III – IVB and no patient had metastatic disease at presentation. Prior to therapy, all patients had biopsy proven squamous cell carcinoma. Full staging included panendoscopy, CT scan of the neck, and CT scan of the chest. In addition, all patients underwent dental evaluation and clearance prior to therapy. Patient characteristics are listed in . The median age was 53.5 years old, 39/42 (93%) were males, 32/42 (76%) were white, and 31/42 (74%) had oropharyngeal primaries. Patients were treated with 3-field head and neck technique (40 patients) or intensity modulated radiation therapy (IMRT) (2 patients). Median dose of 70.4 Gy (range: 68–71 Gy) in a median number of 38 fractions (range: 34–39) at 1.8–2 Gy per fraction was given concurrently with platinum based chemotherapy. Thirty-three patients received q weekly chemotherapy and 9 patients received q 3 weekly chemotherapy. Twenty-seven of 42 (64%) patients received cisplatin/Taxol. The median elapsed treatment time for irradiation was 54 days (range: 47–79). All patients underwent detailed clinical examination and CT scan of the neck after chemoradiotherapy, and PET scan was performed in the later part of the study. All patients had clinical and if necessary, biopsy proven evidence of control of primary tumor prior to ND. The subsets of this analysis had residual nodal disease based on either clinical examination, CT scan, or PET scan. Nodal levels were determined based on multiple clinical examinations performed before and after therapy combined with CT information.
Table I. Patient characteristics
Adjuvant neck dissection was performed at a median of 59 days from the completion of chemoradiotherapy (range: 27–119). Neck dissection consisted of either modified radical neck dissection (MRND) or selective neck dissection (SND) based on surgical evaluation and surgeon's preference for the type of procedure. Intra-surgeon variation was seen on the choice of ND depending on the clinical features and extent of nodal disease before and after completion of chemo-radiotherapy. One of the surgeon (s) always performed MRND. A dedicated pathologist reviewed the neck dissection specimen and his histopathological findings were presented at the multi-disciplinary tumor board. After surgery, patients were followed by the surgeon, radiation oncologist and medical oncologist at least every 3 months for the first two years, every 6 months for the next two years and then yearly thereafter.
Data analysis
For the clinical characteristics of pathologically positive versus pathologically negative patients, Wilcoxon Rank Sum Tests were performed for the comparisons of continuous variables, and Fisher's Exact Tests were performed for the comparisons of categorical variables.
Fisher's Exact Test was performed for comparing the percentage according to sites involved for pathologically node positive versus node negative patients.
Results
The MRND and SND were performed in 26 (58%) and 16 (38%) patients respectively. Six patients underwent bilateral neck dissection. Median number of nodes removed was 14.5 (range: 5–67). Of the 42 patients undergoing neck dissection, 11 (26%) were found to have pathologically positive nodes. The clinical characteristics of the pathologically node positive versus negative patients are similar and seen in . Treatment characteristics of pathologically node positive versus node negative patients were also similar and are depicted in . The percentage according to primary sites for pathologically node positive versus negative patients is seen in . The laryngeal/hypopharyngeal primary sites had higher nodal positivity compared with oropharyngeal/nasopharyngeal primaries.
Table II. Clinical Characteristics of Pathologically Positive versus Pathologically Negative Patients.
Table III. Treatment characteristics of Pathologically Positive versus Pathologically Negative Patients.
Table IV. Percentage According to Sites Involved for Pathologically Node Positive versus Node Negative Patients
The involved pathological levels were always confined within the clinically documented persistent nodal disease (). For patients with pathologically positive nodes, the median number of positive nodes was 1 (range: 1–9). Six patients had one positive node, 3 patients had 2 positive nodes, 1 patient had 3 positive nodes and 1 patient had 9 positive nodes.
Figure 1. The clinical and pathological nodal level(s) involved in patients with positive neck dissections revealing pathologic positively always within the clinically involved nodes.
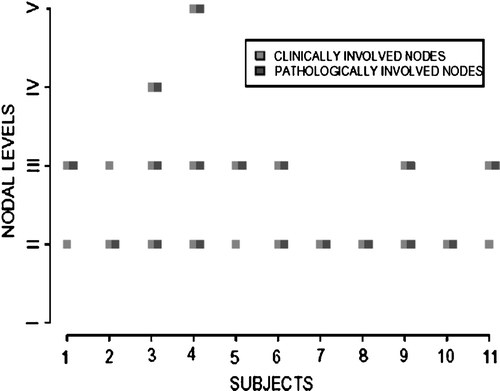
Analysis of 48 hemi neck dissections separately revealed pathological positivity in 13 (27%) necks. The percentage of positive nodes to total nodes removed in 13 positive hemi necks ranged between 2–25% (median 10%). Six of 13 positive hemi necks (46%) had only microscopic or minute viable cancer among positive node(s) removed.
Morbidity after ND was evaluated for all patients except those with recurrent disease or metastases. Sixteen patients had decreased range of motion (ROM) or pain of the neck or shoulders, 12 underwent physical therapy (PT), and six patients had wound healing problems. The latter problem was seen exclusively in patients undergoing MRND. The median follow up time from the neck dissection for the entire group of patients was 278.5 days (range: 9–1 167). Sixty-four percent (7/11) of the pathologically node positive patients were free of disease and 97% (30/31) of pathologically negative patients were free of disease. Three patients developed local failure and distant failure at the same time, one patient developed regional failure, and one patient died of metastases from biopsy proven colorectal carcinoma. One patient developed a new primary of the hypopharynx and underwent surgical resection with ND and is without evidence of disease.
Discussion
Concurrent platinum based CRT is now the preferred treatment for nonsurgical management of locally advanced squamous cell carcinoma of the head and neck. Recent trials have not only demonstrated improved local control but also a survival advantage with organ preservation strategies comparing CRT to RT alone Citation[1], Citation[2]. There is also the added benefit of improved quality of life due to infrequent need for surgical removal of these vital organs. There has been a trend to reduce the treatment related morbidity while maintaining excellent control rates with different strategies including limiting the role of surgery following CRT. Although considered standard following RT alone, the role of ND has been questioned since incorporating CRT as standard treatment Citation[4], Citation[6], Citation[8–10]. Investigators in general agree to observe rather than perform ND in patients achieving nodal CR after completion of CRT in spite of debate on this issue. A recent report further supports this argument not to perform ND after complete clinical response Citation[11]. On the other hand, most would argue to perform ND when there is clinical or radiological evidence of persistent abnormality following CRT.
Several questions remain to be answered to optimize and incorporate surgical modality judiciously following CRT. The necessity of ND based on clinical or radiological response remains to be determined. Due to lack of randomized data, there is no unanimity on indications, timing and type of ND. By convention, ND is usually performed within 4–8 weeks of completion of chemo-radiation. This issue is further complicated by recent reports utilizing PET scans for selecting patients with residual viable cancer who would benefit from ND Citation[17]. The sensitivity and specificity of PET depends on the duration between scan and completion of therapy Citation[18]. In our study, PET was done in small number of patients and that too within short duration of completing treatment thereby limiting its value. Routine use of PET at appropriate time interval should help to further select patients for ND by decreasing the clinical false positive persistent disease. Many studies showing higher incidence of pathologic persistent disease either received radiotherapy alone or lower doses of RT to neck concurrent with chemotherapy Citation[6], Citation[13]. A strategy to perform ND after RT alone made sense in the past; but excellent control rates with CRT make this issue debatable. It appears that an excessive number of nodes were removed during ND given that more than 75% of nodes removed in the present study were always without evidence of viable cancer. In addition, nodal positivity was never seen outside the clinically documented residual disease. There are no studies in literature analyzing quantitatively the extent and number of positive nodes or providing in-depth information on the ratio of number of nodes removed during ND to viable cancer detected or a temporal relationship of pathologic positivity to the anatomic site of persistent disease. There is no information on clinical outcome based on type of ND. Use of MRND after CRT can have significant complication rate Citation[16]. Our data indirectly support that limited ND may be equally effective with reduced morbidity. This has to be viewed with caution due to retrospective nature of our study as well as to limitations to accurately quantify ND specimens pathologically and correlate to clinical persistent disease. The level designation can be arbitrary and not based on clear demarcations. Robbins et at recently published a large study with similar conclusions and even recommending super selective ND for patients with residual disease confined to one level after CRT (in this case, intraarterial chemotherapy was used. Citation[19]. Therefore, use of SND or even gross nodal dissection could significantly alter toxicity profile of these patients thereby impacting their quality of life.
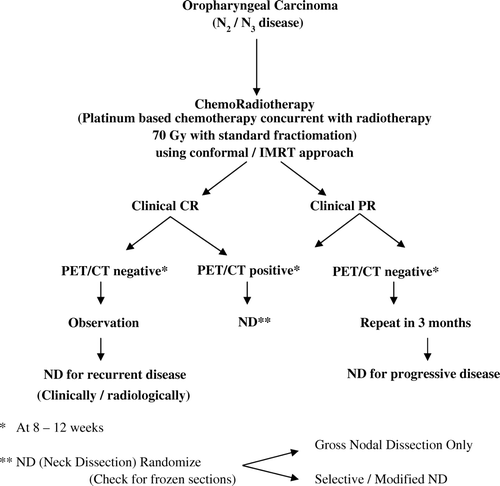
Although literature specifically addressing ND after CRT is lacking, it has been generally assumed that all patients with persistent disease should undergo MND. Some studies do make a case for observation after complete clinical response to CRT; and, planned ND in patients with partial response is supported by the fact that residual cancer is found in ND specimens of a significant percentage and regional failure is high. Our study does not challenge this assumption based on 25% pathologic positivity, however, limited ND needs to be explored in this setting. There is no literature on this particular issue. In our study, pathologic responders after therapy were significantly higher in oropharynx/ nasopharyngeal primaries compared with larynx/hypopharynx primary sites. The proposed trial () should be limited to oropharynx and nasopharyngeal primaries only, due to these sites being exquisitely sensitive to CRT; therefore, any potential effect of limited ND is less likely. There is emerging data integrating information on clinical and radiological (including PET scan) response to therapy with the pathologic outcome. The final answer however would emerge from a randomized trial. The trial should incorporate randomization based on type of ND and stratified on response to therapy.
Figure 2. The suggested possible schema for a trial to optimize the use of imaging modalities for selecting patients for surgical intervention. The recommended design of the trial will test if gross nodal dissection (could also be termed as “supra selective ND”) has similar outcome as standard ND in terms of treatment efficacy with reduced mobility.
Conclusion
We question the role of standard modified radical neck dissection for persistent nodal disease following definitive chemo-radiotherapy. Due to paucity of literature on this issue, the question would be best answered by a randomized trial which should also incorporate conventional and functional imaging modalities to optimize the surgical intervention. The ultimate aim would be to limit treatment related morbidity without impacting tumor control.
References
- Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999; 91: 2081–6
- Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–8
- Barkley HT, Fletcher GH, Jesse RH, Lindberg RD. Management of cervical lymph node metastases in squamous cell carcinomas of the tonsillar fossa, base of tongue, supraglottic larynx and hypopharynx. Am J Surg 1972; 124: 462–7
- Armstrong J, Pfister D, Strong E, Heimann R, Kraus D, Polishook A, et al. The management of the clinically positive neck as part of a larynx preservation approach. Int J Radiat Oncol Biol Phys 1993; 26: 759–65
- Parsons JT, Mendenhall WM, Stringer SP, Amdur RJ, Hinerman RW, Villaret DB, et al. Squamous cell carcinoma of the oropharynx: Surgery, radiation therapy or both. Cancer 2002; 94: 2967–80
- Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy-the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys 1986; 12: 733–40
- Weisman RA, Robbins KT. Management of the neck in patients with head and neck cancer treated by concurrent chemotherapy and radiation. Otolaryngology Clin North Am 1998; 3: 773
- Narayan K, Crane CH, Kleid S, Hughes PG, Peters LJ. Planned neck dissection as an adjunct to the management of patients with advanced neck disease treated with definitive radiotherapy: For some or for all?. Head Neck 1999; 21: 606–13
- McHamm SA, Adelstein DJ, Rybicki LA, Lavertu P, Esclamado R, Wood BG, et al. Who merits a neck dissection after definitive chemoradiotherapy for N2–N3 squamous cell head and neck cancer?. Head Neck 2003; 25: 791–8
- Stenson KM, Haraf DJ, Pelzer H, Recant W, Kies MS, Weichselbaum RR, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy-the feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg 2000; 126: 950–6
- Goguen LA, Posner MR, Tishler RB, Wirth LJ, Norris CM, Annino DJ, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2006; 132: 526–31
- Wanebo H, Chougule P, Ready N, et al. Surgical resection is necessary to maximize tumor control in function-preserving, aggressive chemoradiation protocols for advanced squamous cancer of the head and neck (stage III and IV). Ann Surg Oncol 2001; 8: 644–50
- Lavertu P, Adelstein DJ, Saxton JP, Secic M, Wanamaker JR, Eliachar I, et al. Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck 1997; 19: 559–66
- Frank DK, Hu KS, Culliney MD, Persky MS, Nussbaum M, Schantz SP, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope 2005; 115: 1015–20
- Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, Downey MA, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1418–23
- Kutler DI, Patel SG, Shah JP. The role of neck dissection following definitive chemoradiation. Oncology 2004; 18: 993–8
- Yao M, Graham MM, Smith RB, Dornfeld KJ, Skwarchuk M, Hiffman HT, et al. Value of FDG PET in assessment of treatment response and surveillance in head and neck cancer patients after intensity modulated radiation treatment: A preliminary report. Int J Radiat Oncol Biol Phys 2004; 60: 1410–8
- Greven KM, Williams DW 3rd, McGuirt WF Sr., Harkness BA, D'Agostino RB, Jr, Keyes JW, Jr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck 2001; 23: 942–6
- Robbins KT, Doweck I, Samant S, Vieira F. Effectiveness of superselective and selective neck dissection for advanced nodal metastases after chemoradiation. Arch Otholaryngol Head Neck Surg 2005; 131: 965–9