Abstract
Purpose. We retrospectively evaluated the impact of percent positive axillary nodal involvement on the therapeutic outcomes in patients with non-metastatic breast cancer receiving postmastectomy radiotherapy and chemotherapy. Materials and methods. Between January 1994 and December 2002, the medical records of 939 eligible non metastatic breast carcinoma patients were analyzed. Chest wall radiotherapy was indicated in case of positive surgical margin, tumor size equal or more than 4 cm, skin-fascia invasion. Lymphatic irradiation was applied for more than three metastatic axillary lymph nodes, incomplete axillary dissection (<10 lymph nodes), extracapsular extension or perinodal fat tissue invasion. A total dose of 50 Gy was given to chest wall and lymph node regions with 2 Gy daily fractions. Statistical analyses were performed by Kaplan-Meier method, Log-rank test and Cox's regression analysis. Results. The median follow-up for all patients alive was 62 months. The 5-year overall survival (OS) and disease-free survival (DFS) for entire cohort were 81%, and 65%, respectively. Univariate analysis for OS revealed significance for tumour size (≤5 cm vs. >5cm, p<0.001), metastatic nodal involvement (0 vs. 1–3 vs. >4 LN, p<0.001), percent positive nodal involvement ([metastatic nodes/total nodes removed]×100; 0 vs.≤25% vs. 26–50% vs. >50%, p<0.001), surgical margin status (negative vs. positive, p=0.05), and hormonal treatment (present vs. absent, p=0.03). DFS had similarly significance for age (≤40 years vs. >40 years, p=0.006), tumour size (0.02), metastatic nodal involvement (p<0.001), percent positive nodal involvement (p<0.001), and perinodal invasion (present vs. absent, p=0.01). Multivariate analysis revealed significance for tumour size, percent positive nodal involvement, hormonal treatment, and surgical margin status for OS. Age and percent positive nodal involvement were found to be significant for DFS. Conclusion. Percent positive nodal involvement was found to be a significant prognostic factor for survival in all end-points.
In patients with invasive breast carcinoma who had been subjected to mastectomy, the majority of the recommendations concerning axillary radiotherapy are based on the number of positive nodes. However this number is to some extent dependent on the number of nodes removed during the axillary dissection Citation[1]. The number of nodes removed during axillary dissection has been demonstrated to have a significant impact not only on regional nodal failure but also on local failure Citation[2–6]. According to Eastern Cooperative Oncology Group (ECOG) data, axillary dissection with 6 lymph nodes removed can give adequate information when there were no metastases Citation[4]. After inadequate axillary dissection (less than 6 nodes removed) both regional control and overall survival rates was shown to be significantly reduced Citation[2]. Axillary failure on the other hand was very rare even in the absence of radiotherapy, if the median number of nodes removed was >12 without any metastatic foci Citation[4], Citation[7]. However the most significant prognostic factor predicting the locoregional recurrence (LRR) is generally believed to be the number of metastatic lymph nodes (LNs). Retrospective analysis of 4 ECOG studies revealed locoregional recurrence rates as 13% when there was 1–3 LN metastases and 29% when more than 3 LN metastases in 2 016 patients who were treated with systemic chemotherapy but no radiotherapy after surgery Citation[4]. The corresponding locoregional recurrence rates in 5 NSABP trials were 13% when there was 1–3 LN metastases, 24% when more than 3 LN metastases Citation[8]. It was reported to be 32% when there were 10 or more LN metastases. Today postoperative adjuvant radiotherapy is generally recommended when there are less than 10 lymph nodes removed or when there are more than 3 metastatic LNs Citation[9–11].
It is suggested that the percent positive axillary nodal involvement represents a more precise predictor of axillary failure and survival than the absolute number of positive nodes Citation[12]. Several studies tested this hypothesis in heterogeneous group of patients with invasive breast carcinoma treated by either breast conserving surgery or mastectomy some receiving radiotherapy and the percentage of involved nodes was found to be useful to select patients for axillary irradiation Citation[12–15]. However, none of them tested this proposition in a group of patients who had high risk features and received post mastectomy radiotherapy and chemotherapy.
In this context, we retrospectively evaluated the impact of percent positive axillary nodal involvement on the therapeutic outcomes in patients with non-metastatic breast cancer receiving post mastectomy radiotherapy and chemotherapy in our institution. Our specific focus was to determine whether the percent positive nodal involvement has an impact on loco-regional control, overall (OS) or disease free survivals (DFS) and to evaluate whether there was a cut of rate in order to discriminate high risk patients from low risk ones.
Methods and materials
In this retrospective study, the medical records of 1 086 non metastatic breast carcinoma patients treated between January 1994 and December 2002 were analyzed. Among them 939 patients at least 6 LNs removed formed this analysis. Chest wall radiotherapy was indicated in case of positive surgical margin, tumour size of >4 cm, skin-fascia invasion. Lymphatic irradiation was added to chest wall radiotherapy when there was more than 3 metastatic axillary lymph nodes, incomplete axillary dissection (<10 LNs), extra capsular extension or perinodal fat tissue invasion. A total dose of 46–50 Gy, given in 23–25 fractions over a period of 5 weeks, was delivered to the whole chest wall. When treated, the supraclavicular fossa received a dose of 50 Gy prescribed at 3 cm through an anterior supraclavicular–axillary field. A posterior axillary field was routinely added. In patients with inner quadrant tumours of >4 cm and/or more than 50% axillary nodal involvement internal mammary lymph nodes were also irradiated. The form of chemotherapy was 6–8 cycles of cyclophosphamide, adriamycine, 5-fluorouracil (CAF) in 487 patients and cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in 251 patients. The other schedules were as CA (Cyclophosphamide, Adriamycine) in 50 patients or CE (Cyclophosphamide, Epirubicine) and Docetaxel in 64 patients or 6–8 courses of CNF (Cyclophosphamide, Vinorelbine, 5-Fluorouracil) in 13 patients. Chemotherapy was given as a neoadjuvant setting in 122 patients, and as an adjuvant setting in 743 patients.
The type of surgery was as modified radical mastectomy in 811 patients, simple mastectomy with axillary dissection in 54 patients, or radical mastectomy in 74 patients. When possible, re-excision was performed if the surgical margins were not free of disease. Despite all efforts, surgical margin was positive in 13 patients. In case of margin positivity and no re-excision were performed, radiotherapy dose was 60 Gy instead of 50 Gy.
Statistical analysis
Primary end points were overall and disease free survival rates. Follow-up time was calculated as the time interval between the date of diagnosis and the date of the first event or the date of last contact with the patient. Statistical analyses were performed by Kaplan-Meier method, Log-rank test and Cox's regression analysis. Statistical differences between groups were calculated using the log-rank test for the potential prognostic factors. The relation of the percent positive nodal involvement to the endpoints was also evaluated using Cox proportional hazards models. Due to co linearity, separate models were run with percent positive nodal involvement and total number of involved nodes. Since the two models are close and implicitly nested, the change in -2 log-likelihood was used along with the change in degrees of freedom, in order to compare models and assess superiority of percent positive nodal involvement. All data analyses were performed using SPSS 11.5 statistical software (SPSS Inc, Chicago, IL).
Results
A total of 939 eligible patients did receive regional irradiation and were available for this analysis. The median age for all patients was 47 years (ranged 19–84 years). Of 939 patients, 509 patients were premenopausal and 430 were postmenopausal. Patients’ characteristics are shown in .
Table I. Clinical and pathological features of patients.
The median number of excised axillary nodes was 18 (ranged, 6 to 74 nodes). Of 939 patients, 257 patients had no lymph node metastases, 263 patients had 1–3 positive nodes, and 419 had >3 positive nodes. The median percent positive axillary involvement was 18%. For the group of patients with 1–3 positive nodes, the median percentage was 10%. It was much higher, 50%, for the group with >3 positive nodes.
The median follow-up for all patients alive was 62 months (Range, 6–137 months). The 5-year OS and DFS for the entire cohort were 81%, and 65%, respectively.
Univariate analysis for OS revealed significance for tumour size (≤5 cm vs. >5cm, p < 0.001), metastatic nodal involvement (0 vs. 1–3 vs. ≥4 LN, p < 0.001), percent positive nodal involvement ([metastatic nodes/total nodes removed]×100; 0 vs. ≤25% vs. 26–50% vs. >%50, p < 0.001) (), surgical margin status (negative vs. positive, p = 0.05), and hormonal treatment (present vs. absent, p = 0.03) ().
Figure 1. Kaplan-Meier curves for overall survival according to percent positive axillary nodal involvement.
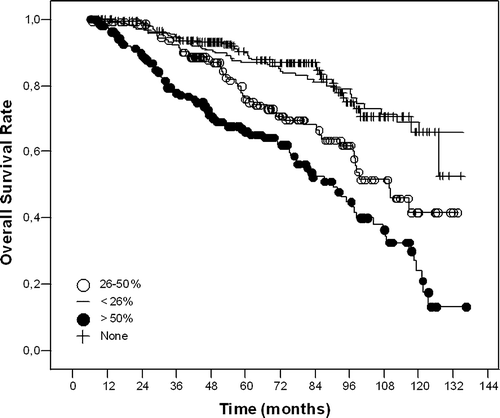
Table II. Univariate analyses for overall survival.
DFS had similarly significance for age (≤40 years vs. >40 years, p = 0.006), tumour size (0.02), metastatic nodal involvement (p < 0.001), percent positive nodal involvement (p < 0.001) (), and perinodal invasion (present vs. absent, p = 0.01) ().
Figure 2. Kaplan-Meier curves for disease free survival according to percent positive axillary nodal involvement.
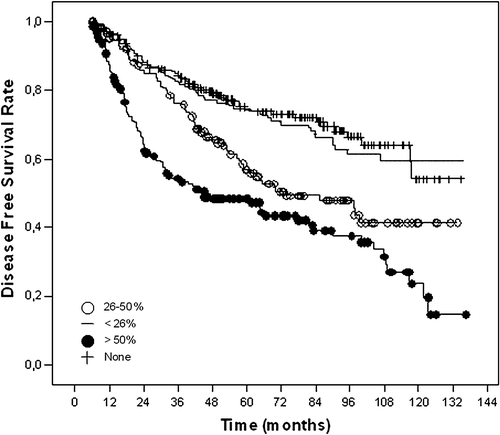
Table III. Univariate analyses for disease free survival.
Multivariate analysis revealed significance for percent positive nodal involvement, tumour size, hormonal treatment, and surgical margin status for OS (). Percent positive nodal involvement and age on the other hand were found to be significant for DFS ().
Table IV. Multivariate analysis for overall survival.
Table V. Multivariate analysis for disease free survival.
Patterns of failure for patients with 50% or more axillary nodal involvement were shown in . The most frequent patterns of failure were distant metastasis for those patients (81 relapse of 106 failures, 76%).
Table VI. Patterns of failure for patients with 50% or more axillary lymph node metastasis.
Discussion
The role of the percent positive lymph nodes in predicting distant metastasis and survival was recently demonstrated in several institutional series Citation[13–18]. Although those studies differed in patient selection, follow-up, and type of surgery and adjuvant therapies, they showed consistently that the percentage of positive lymph nodes is a significant independent prognostic indicator of survival in women with lymph node-positive breast carcinoma. In some of these series, patients did not receive radiotherapy at all, or received radiation therapy after breast conserving surgery Citation[13], Citation[14], Citation[16], Citation[18]. One of the distinctive features of our study from those series is that all our patients received radiotherapy according to our institutional criteria. Secondly, we excluded patients treated with breast conserving surgery and only analyzed the results of patients with mastectomy and adequately treated axilla. Consequently the current series was based on a relatively large group of patients, who were homogeneously treated in a single institution. For these reasons, we believe that this data might provide a different point of view on the prognostic role of the percent positive axillary nodal involvement in the presence of post mastectomy radiotherapy.
Voordeckers et al. analyzed the OS and cause-specific survival rates as a function of the percent positive lymph nodes in 741 patients with node-positive disease treated with breast-conserving therapy or mastectomy, 97% of whom received radiotherapy Citation[14]. In their multivariate analysis, the percentage of positive lymph nodes was the most significant factor for survival. Similar to our findings, the total number of positive lymph nodes lost significance when the percentage of positive lymph nodes was taken into account. Truong et al, reported the prognostic significance of the percentage positive axillary lymph nodes in 542 T1-2 breast cancers with 1–3 positive lymph nodes Citation[13]. They suggested that patients with more than 25% positive lymph nodes may be appropriate candidates for trials that evaluate novel systemic therapy strategies with newer agents and varying dose intensities to optimize systemic disease control. In an analysis of 453 patients with Stage I and II breast carcinoma who underwent either breast-conserving therapy or mastectomy, van der Wal et al. found that, among patients with positive lymph node status, age, the number of dissected lymph nodes, and the percentage of positive lymph nodes were significant prognostic factors for survival Citation[16]. These findings agree with those of a smaller series from Megale Costa et al. of 168 patients who had a short mean follow-up of only 26 months Citation[15]. In a combined analysis of M.D. Anderson Cancer Centre and British Colombia series, Woodward et al. revealed the risk of LRR as 9–15% for <10% nodal ratio and 18–25% for 10–20% nodal ratio in patients who had undergone to mastectomy without adjuvant radiotherapy Citation[18]. In our study patients with ≤25% LN involvement showed favourable prognosis compared to the others showing higher LN involvement. Five year DFS rates in patients with ≤25% seem to behave similar to N0 patients when adequately treated. However it should be kept in mind that all the patients in this series were irradiated after mastectomy and received systemic therapy as chemotherapy and or hormonotherapy.
Adjuvant radiotherapy after mastectomy is usually recommended in patients with more than 3 LN metastases Citation[9], Citation[11], Citation[19], Citation[20]. However the update analysis of Danish studies demonstrated that postoperative radiotherapy produced significant improvement both in DFS and OS not only in patients with 1–3 LN metastases but also patients with ≥4 LN metastases Citation[21]. Twenty-year results of British Colombia trial showed similar results demonstrating significant survival advantages when radiotherapy was added to patients both 1–3 and ≥4 LN metastases Citation[22]. In addition the recent meta-analysis by Early Breast Cancer Trialists Collaborative Trialists’ Group revealed that adjuvant radiotherapy after mastectomy decreased 5 year local risk from 16 to 4% in patients with 1–3 LN metastases Citation[23]. In a cohort of 5 996 patients, Tai et al. showed 25% involved lymph node ratio (LNR) as a cut-off point Citation[17]. In that retrospective analysis, the authors demonstrated that regional radiotherapy significantly reduced the chance of any first recurrence in patients with 25–75% LNR but did not make a difference in patients with ≤25% LNR. The cut-off point in our series is 25% similar to Tai et al.'s and patients with ≤25% LNR showed more favourable outcome without any statistical difference than patients with N0 disease. So we think that it would be beneficial to change the guidelines in terms of radiotherapy indications and when considering as a significant prognostic factor, LNR can also be used for radiotherapy indication.
Differences in axillary lymph node staging techniques, particularly the extent of axillary dissection have the potential to impact loco-regional disease control Citation[9], Citation[19]. In general, it has been suggested that the Level I and II lymph nodes should be removed for precise staging Citation[24–26]. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 study, the estimate of quantitative lymph node status (1–3 vs. ≥4 positive lymph nodes) was more consistent when at least ten lymph nodes were removed Citation[27]. The Danish Breast Cancer Cooperative Group suggests that the number of lymph nodes removed with Level I and II dissection should be at least ten to exclude down-staging of patients with positive lymph nodes as lymph node-negative Citation[24]. In view of that, the Danish randomized trials have been criticized due to limited axillary surgery removing a median of seven lymph nodes, compromising staging precision and regional disease control Citation[28], Citation[29]. It is noteworthy that, the median number of dissected lymph nodes in our series was 18, much higher than many reported series, suggesting the accuracy of axillary staging in this large cohort.
Fortin et al. argued the use of percent positive axillary lymph node involvement in the management of patients with 1–3 positive nodes since the percentage will always be lower particularly in this group after a complete dissection Citation[30]. A percentage of higher values can be expected to occur only for patients with an incomplete dissection. Nevertheless, this is a very rare condition that occurred in only 4% of patients in our series (34 patients with >25%). In this context, it can be expected that the percent positive axillary involvement will not be useful in N1-3 group if they benefit from regional radiotherapy. However, the percentage of involved nodes in patients with more than 3 positive nodes can change their management. In our series survival is worse when ≥50% of nodes is involved and this was relatively frequent, with 22% of more than 3 positive nodes patients having a percentage of ≥50% involved nodes. Therefore, management can be expected to change based on the percentage of involved nodes, as opposed to solely the number of positive nodes determining recommendations. Since these patients with more than 3 positive lymph nodes are already potential candidate for regional radiotherapy, more aggressive systemic regimens should be searched.
In conclusion, we did demonstrate percent positive axillary nodal involvement predicts survival in non-metastatic post mastectomy breast cancer patients receiving regional radiotherapy. This prognostic factor can be used as a decision tool in the management of patients after post mastectomy radiotherapy, in addition to the other well known prognostic factors for recurrence. It seems that more efficient therapeutic approaches are certainly needed for patients with more than 50% axillary nodal metastases.
This manuscript was presented in the 14th Annual European Society of Medical Oncology meeting (ESMO).
References
- Wong JS, O'Neill A, Recht A, et al. The relationship between lymphatic vessel invasion, tumor size, and pathologic nodal status: Can we predict who can avoid a third field in the absence of axillary dissection?. Int J Radiat Oncol Biol Phys 2000; 48: 133–7
- Grills IS, Kestin LL, Goldstein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: Regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys 2003; 56: 658–70
- Fowble B, Solin LJ, Schultz DJ, et al. Frequency, sites of relapse, and outcome of regional node failures following conservative surgery and radiation for early breast cancer. Int J Radiat Oncol Biol Phys 1989; 17: 703–10
- Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: Experience of the Eastern Cooperative Oncology Group. J Clin Oncol 1999; 17: 1689–700
- Galper S, Recht A, Silver B, et al. Is radiation alone adequate treatment to the axilla for patients with limited axillary surgery? Implications for treatment after a positive sentinel node biopsy. Int J Radiat Oncol Biol Phys 2000; 48: 125–32
- Galper S, Recht A, Silver B, et al. Factors associated with regional nodal failure in patients with early stage breast cancer with 0-3 positive axillary nodes following tangential irradiation alone. Int J Radiat Oncol Biol Phys 1999; 45: 1157–66
- Fowble B. Postmastectomy radiation in patients with one to three positive axillary nodes receiving adjuvant chemotherapy: An unresolved issue. Semin Radiat Oncol 1999; 9: 230–40
- Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol 2004; 22: 4247–54
- Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001; 19: 1539–69
- Shenkier T, Weir L, Levine M, et al. Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. Cmaj 2004; 170: 983–94
- Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16: 1569–83
- Katz A, Buchholz TA, Thames H, et al. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: Implications for adjuvant irradiation. Int J Radiat Oncol Biol Phys 2001; 50: 397–403
- Truong PT, Berthelet E, Lee J, et al. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer 2005; 103: 2006–14
- Voordeckers M, Vinh-Hung V, Van de Steene J, et al. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol 2004; 70: 225–30
- Megale Costa LJ, Soares HP, Gaspar HA, et al. Ratio between positive lymph nodes and total dissected axillaries lymph nodes as an independent prognostic factor for disease-free survival in patients with breast cancer. Am J Clin Oncol 2004; 27: 304–6
- van der Wal BC, Butzelaar RM, van der Meij S, et al. Axillary lymph node ratio and total number of removed lymph nodes: Predictors of survival in stage I and II breast cancer. Eur J Surg Oncol 2002; 28: 481–9
- Tai P, Yu E, Sadikov E, et al Prognostic value of nodal ratios (NR) in node positive breast cancer patients. Int J Radiat Oncol Biol Phys 2006;66(3): Supplement, abstract no: 12.
- Woodward WA, Truong PT, Thames O, et al Using the nodal ratio of positive/excised nodes rather than the absolute number of positive lymph nodes minimizes differences in postmastectomy LRR relapse risk between the British Colombia (BC) and the University of M.D. Anderson Cancer Center (MDACC) prospective data sets. Int J Radiat Oncol Biol Phys 2006;66(3): Supplement, abstract no: 10.
- Truong PT, Olivotto IA, Whelan TJ, et al. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. Cmaj 2004; 170: 1263–73
- Recht A, Edge SB. Evidence-based indications for postmastectomy irradiation. Surg Clin North Am 2003; 83: 995–1013
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to the patients with 4 or more positive nodes, as recommended in the international consensus reports? A subgroup analysis of the DBCG 82b and c randomized trials. Radiother Oncol 2004;73(Suppl 1): abstract no: 33.
- Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005; 97: 116–26
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005; 366: 2087–106
- Axelsson CK, Mouridsen HT, Zedeler K. Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer 1992; 28A: 1415–8
- Iyer RV, Hanlon A, Fowble B, et al. Accuracy of the extent of axillary nodal positivity related to primary tumor size, number of involved nodes, and number of nodes examined. Int J Radiat Oncol Biol Phys 2000; 47: 1177–83
- Mersin H, Yildirim E, Bulut H, et al. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. Eur J Surg Oncol 2003; 29: 132–8
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347: 567–75
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Fortin A, Dagnault A, Blondeau L, et al. The impact of the number of excised axillary nodes and of the percentage of involved nodes on regional nodal failure in patients treated by breast conserving surgery with or without regional irradiation. Int J Radiat Oncol Biol Phys 2006; 65: 33–9