Abstract
A postmenopausal lady with an in situ pacemaker developed a lump in the left breast and was diagnosed to have breast cancer. The patient underwent breast conservative surgery and was planned for post operative radiotherapy. The location of the tumor relative to the pacemaker provided a unique challenge in planning radiotherapy and the patient had an uneventful post radiotherapy course. A literature review revealed that modern generation pacemakers are very sensitive to radiation compared to their older counterparts. The present article makes suggestions towards reducing dose in radiotherapy planning in pacemaker patients.
With more than one million new cases each year, female breast cancer is the second most common cancer in the world and the most common cancer among women Citation[1]. Breast conserving therapy (BCT) is increasingly being integrated into the management of breast cancer. The obvious advantages of BCT are an equivalent disease free and overall survival rate as compared to mastectomy with preservation of the breast Citation[2], Citation[3]. Over the past decades, there has been an increase in the average life expectancy rate throughout the world, albeit with comorbidities such as diabetes, hypertension and strokes Citation[4]. One of the common conditions in elderly age group includes conduction defects in the heart secondary to ischemic heart disease Citation[5]. Often this necessitates the placement of a pacemaker to regulate the electrical activity of the heart. A postmenopausal lady with an in situ pacemaker developed left sided breast cancer. The patient underwent breast conservative surgery (BCS) and was planned for post operative radiotherapy. The location of the tumor relative to her pacemaker provided a unique challenge in planning radiotherapy. In this article we are discussing the issues in radiotherapy planning of this patient with an in situ pacemaker and presenting a review of the current literature.
Case report
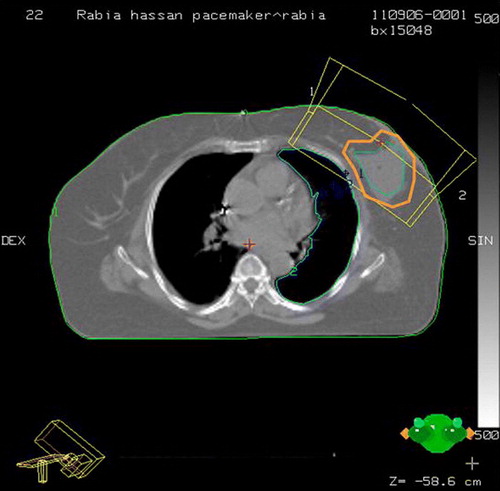
A 64-year-old postmenopausal multiparous lady, known diabetic and hypertensive, was investigated for repeated episodes of giddiness and hypertension refractory to antihypertensives. She was detected to have a 2:1 heart block with right bundle branch block and left anterior hemi block. She subsequently underwent permanent pacemaker placement (St Jude Pacemaker; Affinity Model DR 5330). Three years later, she presented with history of lump in the left breast of 3 months duration. On clinical examination there was a 2.5×2.5 cm hard lump in upper outer quadrant of the left breast. FNAC was positive for ductal carcinoma. Echocardiography before surgery was suggestive of concentric left ventricular hypertrophy (LVH) with diastolic dysfunction and a left ventricular ejection fraction (LVEF) of 50%. Electrocardiogram (ECG) showed a normal pacemaker rhythm. She underwent BCS under close cardiac monitoring by a cardiologist and a pacemaker engineer. The histopathology revealed infiltrating ductal carcinoma grade II with a pathologic tumor size of 2.5×2.5×2.0 cm. All the cut margins were free. Axillary sampling revealed eight negative nodes. The specimen was ER negative, PR positive and cerbB2 negative. Postoperative 2D-echocardiography revealed a LVEF of 50% with no appreciable change as compared to baseline. Cardiology consultation for evaluation of the pacemaker functioning was documented to be normal. In view of her favorable histopathology and her cardiac ailment, adjuvant chemotherapy was not given and she was directly referred for postoperative radiotherapy. For radiotherapy planning, serial CT slices were taken with appropriate immobilization. The acquired images were transferred to the planning system. During planning, it was realized that whole breast radiotherapy would result in direct irradiation of the lower half of the pacemaker due to its location within the radiation portal. Since her clinical and pathological profile fulfilled the current American brachytherapy Society (ABS) and National Surgical Adjuvant Bowel Project (NSABP) criteria for Accelerated Partial Breast Irradiation (APBI), it was decided to leave the upper portion of the breast unirradiated. Her final target volume consisted of the lumpectomy cavity (located in the central and a uniform 2.5 cm margin (with editing towards the chest wall and skin) Citation[6]. In the subsequent radiotherapy portal, the entire target was covered by the tangentials (, central slice) while sparing the pacemaker whose inferior edge remained more than 3 cm away from the upper border of the portal (). The dosimetry of the case was done in the Cadplan planning system (Varian). In view of the large size of the breast, 10 MV x-rays were used in treatment planning with a 15 degree bilateral wedge for appropriate dose distribution. Care was taken to see that besides the pacemaker device, the leads going to the heart were also kept out of the radiotherapy portal. For the first and subsequent few fractions during the course of radiotherapy, continuous ECG monitoring was done during the radiotherapy beam on time. A back-up temporary pacemaker was kept ready for use in case permanent pacemaker malfunction occurred during radiotherapy. The gross ECG pattern before and during radiotherapy did not reveal any change. The parameters of the pacemaker were recorded pre radiotherapy and during the time the radiotherapy beam was on (). Dosimetry with diodes was done on the first day to measure dose at the inferior most edge of the pacemaker (closest part of pacemaker to the upper border of the field (). No event of bradycardia/tachycardia/arrhythmia was recorded. The pacemaker engineer evaluated the pacemaker parameters every week. ECG was done before, during and after completion of course of radiotherapy. By our estimate, the pacemaker received a dose of 15.37 cGy×28 fractions = 4.3 Gy (). The varying depth of the pacemaker from the surface was recorded (). Since our recording was on the surface and in view of our dosimetric findings, we decided to limit the total dose of radiotherapy to 50.4 Gy only at 1.8 Gy per fraction. Also, we did not give boost field with electrons in view of the increased risk of dose scatter with electrons. At 10 months of follow-up, the patient is disease free and the pacemaker is functioning normally.
Figure 2. Digital Reconstructed Radiograph (DRR) showing the pacemaker and the leads in relation to the radiation beam.
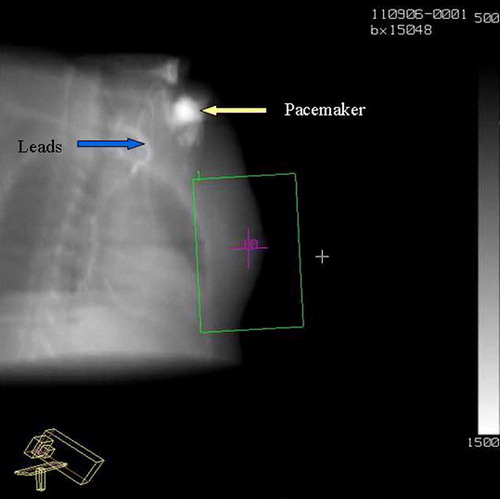
Table I. Pacemaker functioning before and during radiotherapy.
Table II. Dosimetry recording of diode.
Table III. Depth of pacemaker from skin surface at different axial levels (in cm).
Discussion
The device
The pacemaker consists of a main device encased in a seal whose electrodes or leads are threaded through intravenously into the cardiac chamber where it lies in contact with the cardiac musculature. It is made of computer chips and a small but long life (5 to 10 years) battery in a sealed case. It can be single chamber (right ventricle), dual chamber (right atrium and right ventricle) or three chamber (right atrium and both ventricles) pacemaker. It is usually placed in a subcutaneous pocket over the pectoral muscles in the left infraclavicular region, posing a problem for delivering radiotherapy for lymphomas, breast, lung, head and neck, thyroid and esophageal cancers. It may also possess sophisticated programmable software and facilities for recording events.
Certain types of electric and magnetic energy can interfere with the operation of the pacemaker, a phenomenon known as electromagnetic interference (EMI) Citation[7–11]. The devices causing EMI are of two types: 1) Conducting: involving a direct contact of the EM source with body. Examples of this include electrocautery or defibrillators. 2) Radiating: requiring no physical contact. Examples include MRI, PET, lithotripsy and megavoltage radiotherapy delivery ( and ) Citation[10], Citation[11].
Table IV. Sources of electro magnetic interference (EMI) with pacemaker.
Table V. Safe procedures with pacemaker in situ.
The mechanism of damage of a pacemaker during radiotherapy includes: a) Ionization of semiconductor material; b) EMI from linear accelerators; c) Abnormal current flows; d) Changes in threshold voltage Citation[10]. The manifestation of damage includes dropped beats, pacemaker induced tachycardia, runaway rhythm, circuitry failure, pacemaker malfunction (erratic pacing, sensing anomalies) and sudden death. Citation[8], Citation[11–14].
Threshold doses
Older generation pacemaker devices based on bipolar semiconductor were very radioresistant. These tolerated doses from 100 to 460 Gy, well above the therapeutic radiotherapy dose for any cancer Citation[7], Citation[8]. Hence these devices did not pose any hindrance in radiotherapy delivery. Modern generation programmable pacemakers containing the complementary metal-oxide semiconductors (CMOS) are very radiosensitive to megavoltage radiation and are affected by doses as low as 0.15 Gy. Therefore these devices pose therapeutic dilemmas in delivering the standard treatment Citation[9], Citation[10]. Each new model in the market differs from older models in some way, either in design or structure or software program, thereby greatly influencing the radiosensitivity and making comparison difficult.
At present there are no uniform recommendations from the manufacturers and the available recommendations are unreliable. Most of the literature addressing this issue is in the form of single patient case reports or in vitro studies only () Citation[15–23]. The only available recommendations are from the AAPM published in 1994, of which there is no recent official updated report. They recommended that maximum dose to the pacemaker be limited below 2 Gy Citation[24]. However, no recommendation for the effect of dose rate has been made. Until sound recommendations become available, it is the responsibility of the individual radiotherapy departments to identify and develop formal protocols for cancer patients with pacemakers especially in the planning and delivery of radiotherapy. Also the safety of high dose rate brachytherapy in case of implanted pacemaker close to target volume and of electronic portal imaging devices is not known. The St Jude's pacemaker guidelines published in June 1998 state that radiation damage to CMOS circuitry can occur at 2000 rads and that the present SJ pacemakers are tested up to 30 Gy. They recommend a lead apron over the generator as it is always to be kept out of field Citation[25]. Medtronic guidelines recommend a total allowable dose to the pacemaker up to 5 Gy and they also require generator to be moved outside the field Citation[26]. Guidant guidelines have recommended that there is “no safe dose” which can be recommended as total allowable dose Citation[27].
Possible solutions and suggestions
For medium and high-risk patients, alternative treatment modalities such as surgery, hormone therapy or chemotherapy should be pursued if they are equally valid treatment options and appropriate for that patient. gives the risk stratifications of a patient with a pacemaker Citation[9]. Medium and high risk categories require personnel trained in advanced life support and on call cardiac team to tackle emergency. High risk cases also require continuous cardiac monitoring during radiotherapy from an outside room.
Table VI. Risk categories for treatment of pacemaker patients by radiotherapy.
Table VII. Published reports of irradiation in patients with paceemaker.
A baseline ECG should be obtained in all patients with pacemakers. This will help in assessment of pacemaker dependency. However, formal interrogation in a pacemaker clinic may also be required and is probably desirable before commencing radiotherapy.
Simple changes in the treatment plan may reduce the dose to the pacemaker to a low risk level. As literature on interaction of modern radiosensitive pacemakers is limited, only in the form of case-reports, pre-clinical animal or dosimetric studies only, systematic cohort studies to assess the short and long term effect of radiation on the functional integrity of pacemakers are essential. This needs to be accorded a high priority by large cooperative oncology groups since with the inevitable ageing of the population and expanding indications for pacemaker implantation, a considerable proportion of cancer patients will have pacemakers in the future. Surgically shifting the pacemaker to a region of less radiotherapy dose can also be considered.
Radiation planning with pacemaker devices
The low energy, kilovoltage x-rays used in simulation for radiotherapy fluoroscopy simulators and CT scanners are unlikely to cause malfunction of pacemakers and can be safely used for radiotherapy planning Citation[25], Citation[28]. The majority of the patients encountered in the radiotherapy department do not have a pacemaker directly in the beam and therefore a radical change to the prescribed treatment is usually not necessary. The issue of radiation and pacemaker is more relevant in tumors related to breast, lung, esophagus and other thoracic tumors. There has been no demonstrable volume effect with the pacemaker for radiotherapy damage. It needs to be noted that there is no safe radiation threshold for megavoltage radiation to preclude their effect on the pacemakers. The cardinal rule is that radiation dose to the pacemaker is the dose to any part of the device (including the leads). Also there does not appear to be any consistent way to predict how a device will fail or at what radiation dose failure will occur. The following possible suggestions are made to keep dose to the pacemaker as low as possible: a) Considering other treatment options (non radiotherapy) if they are equally efficacious; b) Surgically shifting the pacemaker to a region of less radiotherapy dose (e.g. contra lateral side); c) Excluding pacemaker from radiotherapy portal in case it is decided to give radiotherapy; d) Wherever possible, to calculate the approximate dose to the pacemaker before initiation of treatment. In vivo dosimetry is preferred but is usually difficult.
Radiotherapy technique should involve judiciously using different beam angles including non-coplaner beam arrangement, radiation quality and modality, asymmetric jaws(towards the pacemaker end to reduce the effect of divergent beam), accessories for beam shaping like multileaf collimators, wedges and metal alloy shielding. Specialized techniques in the form of 3DCRT or IMRT can be employed taking the pacemaker as the organ at risk Citation[21]. It seems prudent to restrict the maximum dose to the pacemaker to less than 2 Gy or at least fulfill the individual manufacturer's specified recommendations. It is advisable to avoid using equipment liable to produce electromagnetic interference (EMI) like beam gating, electronic portal vision device (EPID) or in room accessories including breathe control systems. Finally, the pacemaker can be shielded with a block or Multi leaf collimator (MLC). The option of interstitial high dose rate brachytherapy can be used in appropriate instances.
Suggestions for manufacturers
The protective measures, which can be incorporated into the pacemaker, include the introduction of an anti-interference circuit and use of bipolar electrodes, titanium casing and asynchronous stimulation Citation[29]. Other measures include incorporation of a band pass filter, which prohibits the entry of signals that are above or below a certain frequency threshold Citation[29]. The manufacturer should also be asked to provide radiation tolerance information regarding relative sensitivity of the pacemaker to previous models. It has be to noted that not all data would be in the public domain and technological improvements occur all the time. Radiation tolerance data pertaining to a particular model are essential. The make and model of the pacemaker, and the manufacturers’ recommendations regarding safe doses for that particular model of pacemaker should be ascertained before radiation planning.
To summarise, modern day pacemakers are much more sensitive to radiation than their primitive counterparts. As such, radiation oncologist especially ones dealing with breast and thoracic tumors need to be aware of the limitations in radiotherapy planning with a pacemaker in situ. Even after careful planning, dosimetry should be performed whenever possible to assess the actual dose being received by the pacemaker so that timely adjustments in total dose and treatment techniques can be made.
References
- Kamangar F, Dores M, Anderson F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137–50
- Fisher B, Anderson S, Bryant J, Margolese R, Deutsch M, Fisher E. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32
- Mond H, Irwin M, Morllo C, Ector H. The world survey of cardiac pacing and implantable cardioverter defibrillators: Calendar year 2001. Pacing Clin Electrophysiol 2004; 27: 955–64
- Bernstein D, Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol 2001; 24: 842–55
- Patel R, Das R. Image guided breast brachytherapy: An alternative to whole breast radiotherapy. Lancet Oncol 2006; 7: 407–15
- Walz BJ, Reder E, Pastore O, Littman P, Johnson R. Cardiac pacemakers. Does radiation therapy affect performance?. JAMA 1975; 234: 72–3
- Katzenberg A, Marcus I, Heusinkveld S. Pacemaker failure due to radiation therapy. Pacing Clin Electrophysiol 1982; 5: 156–9
- Sundar S, Symonds P, Deehan C. Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Review 2005; 31: 474–86
- Last A. Radiotherapy in patients with cardiac pacemakers. Br J Radiol 1998; 71: 4–10
- Neihaus M, Terbenjohanns J. Electrical interference in patients with implanted pacemakers or cardioverter-defibrillators. Heart 2001; 86: 246–8
- Lee W, Huang K, Mechling E. Runaway atrioventricular sequential pacemaker after radiation therapy. Am J Med 1986; 81: 883–6
- Quertermous T, Megahy S, Das Gupta S. Pacemaker failure resulting from radiation damage. Radiology 1983; 148: 257–8
- Pourhamidi H. Radiation effect on implanted pacemakers. Chest 1983; 84: 499–500
- Lewin A, Serago F, Schwade G, Abitbol A, Margolis S. Radiation induced failures of complementary metal oxide semiconductor containing pacemakers: A potentially lethal complication. Int J Radiat Oncol Biol Phys 1984; 10: 1967–69
- Brooks C, Mutter M. Pacemaker failure associated with therapeutic radiation. Am J Emerg Med 1988; 6: 591–3
- Muller-Runkel R, Orsolini G, Kalokhe P. Monitoring the radiation dose to a multiprogrammable pacemaker during radical radiation therapy: A case report. Pacing Clin Electrophysiol 1990; 13: 1466–70
- Ngu L, O'Meley P, Johnson N, Collins C. Pacemaker function during irradiation: In vivo and in vitro effect. Australas Radiol 1993; 37: 105–7
- Raitt H, Stelzer J, Laramore E, Bardy H, Dolack L, Poole E, et al. Runaway pacemaker during high-energy neutron radiation therapy. Chest 1994; 106: 955–57
- Nibhanupudy R, de Jesus A, Fujita M. Radiation dose monitoring in a breast cancer patient with a pacemaker: A case report. J Natl Med Assoc 2001; 93: 278–81
- Riley B, Gracia J, Guerrero. The utilization of 3-Dimensional noncoplanar treatment plan to avoid pacemaker complications. Med Dosim 2004; 29: 92–6
- Li DM, Xu B, Xu XN. Radiotherapy of tumor patients implanted with permanent cardiac pacemaker: 4 cases report. Ai Zheng 2004; 23: 722–3
- Sepe S, Schaffer P, Krimmel K, Schaffer M. Irradiation treatment of laryngeal cancer in a patient with an implantable cardioverter-defibrillator (ICD). Onkologie 2007; 30: 378–80
- Marbach R, Sontag R, Van Dyk J. Management of radiation oncology patients with implanted cardiac pacemakers: Report of AAPM Task Group No. 34. American Association of Physicists in Medicine. Med Phys 1994; 21: 85–9
- St. Jude Medical Technical Services. Radiation Revision 9/02. Symlar, CA: St. Jude Medical Technical Services; 2000. p 1–3.
- Medtronic USA Inc. Technical services. Radiation and pacemakers. Minneapolis: Medtronic USA Inc.; 2002. p 1–2.
- Guidant Corporation Cardiac Rhythm Management Technical Services. The impact of therapeutic radiation on guidant implantable pacemakers (IPMs) and implantable cardioverter defibrillators (ICDs). Revision L02/13/03, St Paul, MN: Guidant Corporation; 2003. p 1–6.
- Rothig H, Wiedemann D, Herrmann T, Burk G. The functional efficiency of cardiac pacemakers as affected by ionizing radiation. Radiobiol Radiother 1990; 31: 341–50
- Lewin A, Serago F, Schwade G. Electromagnetic interference in cardiac rhythm management devices. AACN Clin Issues 2004; 15: 391–403