To the Editor
In adult patients with medulloblastoma, standard therapy consists of resection with postoperative craniospinal irradiation. The value of chemotherapy remains debatable Citation[1]. Thus, there is so far no evidence-based second-line therapy. Since somatostatin regulates proliferation and differentiation in medulloblastoma cells Citation[2] and may also inhibit angiogenesis Citation[3], somatostatin analogues such as octreotide may be attractive agents to treat patients with relapsed medulloblastoma.
Case report
We describe a case of a 43-year-old female with desmoplastic medulloblastoma in the right cerebellar hemisphere and dorsal mesencephalon. The patient was initially treated with partial resection, craniospinal irradiation (35.3 Gy) with a boost to the posterior fossa (total dose 56 Gy) and adjuvant chemotherapy with vincristin, CCNU and cisplatin (4 cycles). Twenty two months after diagnosis, she noted hemianopsia to the right visual field. MRI revealed a contrast enhancing lesion in the right optic nerve without progression of the primary lesion.
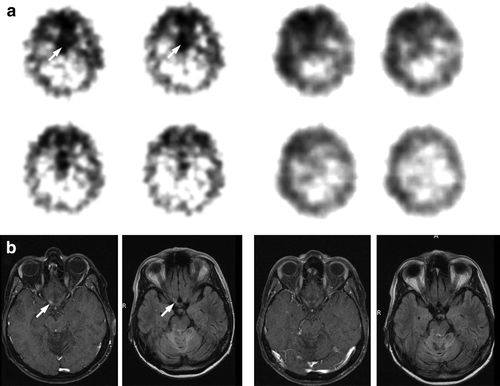
Somatostatin receptor imaging with 111In-octreotide scintigraphy revealed positive receptor status for the optic nerve lesion and a negative status for the primary lesion (A). Due to the high risk of impairment of vision, a second biopsy was not performed. Since no further treatment option was available, we initiated treatment with Octreotide (Sandostatin™) 100 µg/d for day 1–14 s.c. followed by Sandostatin LAR™ (started at day 3; cycle 1–2: 40 mg, 3–4: 60 mg, 5–9: 90 mg/4 weeks i.m.) after written informed consent was obtained from the patient. Twelve months after initiation, the patient showed significant improvement of hemianopsia in perimetry and a sustained complete response of the right optic nerve lesion in MRI (B). The treatment was well tolerated and no adverse events were reported until the 10th cycle, when the patient presented with headache and fatigue. Initial laboratory analyses revealed a Coombs negative haemolytic anemia (Hb 2.8 g/dl, serum lactate dehydrogenase 918 U/l, bilirubin 3.5 mg/dl, haptoglobin <7 mg/dl). Within few days, the thrombocyte count dropped to 34/nl and the peripheral blood smear showed fragmented red cells. Further, a severe deficiency of the von Willebrand factor-cleavage protease ADAMTS13 was found (9%, range 30–120%). Upon treatment with packed red blood cells, fresh frozen plasma and prednisolone 70 mg daily, the patient improved clinically and the pathologic laboratory findings normalized within 14 days (Hb 11 g/dl, thrombocyte count 350/nl). Sandostatin™ treatment was withheld without further relapse so far.
Figure 1. 111In-octreotide-szintigraphy before treatment (4 panels left) and during treatment (4 panels right) with Sandostatin LAR™ (after 4 cycles). Arrows show positive receptor status. B. MRI (T1 plus Gadolinium and FLAIR) before treatment (2 panels left) and during treatment (2 panels right) after 4 cycles with Sandostatin LAR™. Arrows show contrast enhancement (T1) and hyperintensity (FLAIR).
Conclusion
Here we present the first report of a possible complete imaging and partial clinical response of a medulloblastoma lesion upon treatment with a somatostatin analogue. Unfortunately, the patient was diagnosed with a thrombotic thrombocytopenic purpura (TTP) in the course of somatostatin analogue therapy. Whether the observed TTP has to be classified as idiopathic, paraneoplastic or as an adverse effect of the somatostatin analogue cannot be finally decided. So far, no association of TTP and somatostatin analogues or TTP and medulloblastoma have been reported although TTP has been described in conjunction with some other tumors Citation[4]. The observed coincidence of somatostatin analogue therapy for medulloblastoma and TTP should certainly not prevent from further exploring the value of somatostatin analogue therapy in patients with relapsed adult medulloblastoma. Treatment options in this situation are still very scarce and the observed sustained response in the case described here is encouraging and warrants further validation in a clinical trial.
References
- Herrlinger U, Steinbrecher A, Rieger J, Hau P, Kortmann RD, Meyermann R, et al. Adult medulloblastoma: Prognostic factors and response to therapy at diagnosis and at relapse. J Neurol 2005; 252: 291–9
- Fruhwald MC, O'Dorisio MS, Pietsch T, Reubi JC. High expression of somatostatin receptor 2 (sst2) in medulloblastoma: Implications for diagnosis and therapy. Pediatr Res 1999; 45: 697–708
- Florio T, Morino M, Villa V, Arena S, Corsaro A, Thellung S, et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology 2003; 144: 1574–84
- Kwaan HC, Gordon LI. Thrombotic microangiopathy in the cancer patient. Acta Haematol 2001; 106: 52–6