Abstract
Introduction. An at least 3-month expected survival is a common inclusion criterion in cancer treatment trials, including advanced pancreatic cancer phase III studies. Published survival curves for advanced pancreatic cancer however seem to reflect a substantial survival shortfall. We wanted to assess the strength of this observation and search for an explanation by reviewing the literature. Methods. A Medline and EMBASE search was done for chemotherapy or chemotherapy based phase III studies in advanced pancreatic cancer published since 1997. Similar search was done at the American Society's of Clinical Oncology web site for abstracts presented since 2000. Three months mortality was based on the survival curves presented. Results. Fourteen papers and five abstracts met our criteria and are included in our review. Six thousand two hundred and twelve patients participated in these trials and 1 447 (23.3%) died in the first 3-month period. Figures were worse in patients with metastases and poorer performance status. Assuming that most deaths during treatment happened during the first 3-months, cause of death was reported in only 40 cases (2.8%). Progressive cancer was reported as cause of death in 21 of these cases. Less frequent causes of death were reported to be infections, ‘complications of cancer’, thromboembolic events and renal failure. Discussion. Overall treatment-related deaths represent a very small percentage of the deaths happening during the 3-month period, and are unlikely to be under-reported given the Good Clinical Practice oversight of these trials. Progressive cancer is likely to be an important cause of early mortality but given the very select nature of the trial-related population this cannot explain the phenomenon of 3-month early death burden of 23.3%. Our hypothesis, supported by multiple autopsy series, is that early death burden in advanced pancreatic cancer trial patients is likely to be due to under-reported vascular thromboembolic events. Thromboprophylaxis needs to be addressed in future trials.
Worldwide there were an estimated 232 306 new cases of pancreatic cancer diagnosed in 2002, accounting for 2% of all new cancer cases Citation[1]. Pancreatic cancer has the poorest prognosis of all adenocarcinomas in the human body and its incidence rate almost matches its prevalence rate. It is one of the leading causes of cancer related deaths and it is the 4th most common cause of cancer related death in the United States and the 6th in the United Kingdom while only being 11th in incidence Citation[1], Citation[2].
Because of the typically late onset of symptoms only around 15–20% of pancreatic cancer cases are amenable to potentially curative surgical resection. In about 40% of the patients, involvement of the major blood vessels around the pancreas (superior mesenteric artery, celiac axis, superior mesenteric vein-portal vein confluence, inferior vena cava and aorta) diagnosed either preoperatively or perioperatively precludes complete resection of the tumour. These patients are grouped as having locally advanced pancreatic cancer and surgery is not a therapeutic option. They are usually treated with radiotherapy, chemotherapy or both and a median survival between 7 and 11 months is the best achievable Citation[3]. The remaining patients (45%) present with metastases. For these patients chemotherapy or best supportive care, if their general condition is poor, are the treatment options and a median survival of around 6–7 months and of just around 3–4 months respectively is to be expected Citation[4].
These data derive from randomised phase III trials testing various treatments (mostly chemotherapy agents) in patients with advanced pancreatic cancer. One of the inclusion criteria, explicitly stated in many of these studies, is that the participants should have an at least 3-month expected survival. This means that other inclusion criteria usually contain ‘copy-paste’ parameters, commonly used for most advanced cancer-treatment trials, pertaining to performance status and end-organ status that generally are expected to distinguish patients likely to reach this 3-month target. The 3-month endpoint corresponds to a common assessment milestone for many trials that have response as an endpoint. Most imaging response data are conventionally obtained and confirmed at around week 12 it is therefore not a trivial point to identify these patients as survival shortfalls can compromise trial endpoints. While these common criteria tend to be by and large successful for many other cancers (e.g. breast, ovarian, colon), this does not seem to be the case in advanced pancreatic cancer even in the hands of trial-experienced oncologists. Looking at the survival curves of these studies, it seems to us that a significant number of patients die within the first 12 weeks from the day of their inclusion in the studies without ever reaching or concluding the first tumour-response assessment milestone. This was also our experience in phase II trial-work we were involved with Citation[5], Citation[6]. We have called this phenomenon ‘early death burden’, and wanting to get a better picture of it we undertook a systematic review of the recent studies and a discussion of the findings.
Methods
A search in the literature (Medline and EMBASE databases) was done for English-language articles presenting results from phase III studies dealing with chemotherapy or chemotherapy based first line treatment in advanced pancreatic cancer until the end of 2006. A study was eligible for our review if there was active treatment in all arms. At least the treatment in one trial's arm had to be chemotherapy or chemotherapy based. Articles dealing with regional treatment were excluded. We wanted our review to be relevant as much as possible to the current practice and as in 1997 the current standard of treatment was established (gemcitabine by Burris et al.) Citation[7] we limited our search to articles published since that landmark article.
Medline search was done with the MeSH term ‘pancreatic neoplasms /drug therapy’ and it was limited to ‘randomized controlled trials’.
Search in the EMBASE database was done with the headings ‘advanced pancreatic cancer or pancreatic cancer or pancreatic adenocarcinoma or pancreatic carcinoma’ and ‘randomized or randomised or phase III’.
We have extended our search to include the web site of the American Society of Clinical Oncology in which abstracts from the annual meetings or from the Gastrointestinal Cancer Symposiums of the Society are posted. The period that our search covered on the above web site was from 2000 until 2006.
A trial was eligible for our review if its authors specifically stated inclusion of patients with an at least 3-month expected survival or if the authors stated that ‘common criteria used in oncology studies applied’ in their study as well (that means also an at least 3-month expected survival). Studies included had to have survival curves available for scrutiny.
Three month mortality was calculated from the survival curves presented in these studies.
Results
Our Medline search identified 79 articles of which 17 were related to our topic. Three of these 17 articles were excluded from our review as in one the authors explicitly stated that a 2 month expected survival was adequate for patient recruitment Citation[8] and in the other two no survival curves were included Citation[9], Citation[10].
EMBASE search revealed 55 articles, 14 of which were related to our topic but all of these had already been identified through the Medline search.
Regarding our search on the American's Society of Clinical Oncology web site, we identified 14 abstracts. Two of those were excluded as Kaplan-Meier survival curves were not presented Citation[11], Citation[12] and seven further abstracts have not been included as since then the related studies have been published in peer-review journals and they had been identified through our literature search.
Overall 14 papers and five abstracts have been included in our analysis Citation[7], Citation[13–30].
Characteristics of the full papers
As it can be seen in , 14 papers have been reviewed. Twelve had two treatment arms. The trial of 5-fluorouracil versus octreotide had three arms and the trial of gemcitabine versus marimastat four. But as in the first study one survival curve was given for both chemotherapy arms (single agent 5-fluorouracil and 5-fluorouracil plus leucovorin arms) Citation[13] and as in the later study in three arms the same drug (marimastat) was used although at different doses Citation[14], we have decided to present both studies as if they had two arms Citation[14]. Gemcitabine was the treatment in the standard arm in 11 trials Citation[14], Citation[16–25], and 5-fluorouracil in the remaining three Citation[7], Citation[13], Citation[15]. In nine of these the treatment in the experimental arm was a doublet that included the standard treatment Citation[15–17], Citation[19], Citation[20], Citation[22–25], in four it was a different single agent treatment Citation[7], Citation[13], Citation[14], Citation[18] and in one it was a regimen with four cytotoxic drugs Citation[17]. In ten trials the drug or combination tested was chemotherapy Citation[7], Citation[15], Citation[16], Citation[19–25] while in the other four it was the somatostatin analogue octreotide, a matrix metalloproteinase inhibitor (marimastat or BAY 12-9566) or a combination of chemotherapy with a matrix metalloproteinase inhibitor Citation[13], Citation[14], Citation[17], Citation[18].
Table I. Three-month mortality in patients with advanced pancreatic cancer received chemotherapy or chemotherapy based treatment in phase III studies which have been presented as full papers in the literature and are included in our review.
In the 14 studies presented as full papers since 1997, 3 687 patients participated (mean number per study 263). The 3-months mortality varied from 10% (gemcitabine/oxaliplatin arm of the Louvet et al. study) Citation[22] to 51% (octreotide arm of the Burch et al. study) Citation[13] with a mean 3-month mortality of 23.6%.
Characteristics of the abstracts
In the five abstracts included in the review, all but one Citation[30] had two treatment arms (). Gemcitabine was the comparator in all of those. The experimental therapy in four of those was single agent chemotherapy or a combination of cytotoxic drugs Citation[26], Citation[28–30]. In the fifth study the combination of gemcitabine with the targeted drug erlotinib was tested Citation[27].
Table II. Three-month mortality in patients with advanced pancreatic cancer treated with chemotherapy or chemotherapy based regimens in phase III studies which have been presented only as abstracts so far and are included in our review.
In the above five studies, 2 525 patients participated (mean number per study 505). The incidence of premature death varied from 13% (gemcitabine arm of Herrmann et al. study) Citation[29] to 30% (gemcitabine/5-fluorouracil arm of the Riess et al. study) Citation[28]. The mean 3-month mortality rate was 22.7%.
Three-month mortality in both full papers and abstracts
Combining results from full papers and abstracts, the mean 3-month mortality was 23.3%. The average 3-month mortality for patients receiving single agent gemcitabine, which is currently the treatment of choice and was the standard treatment for most studies reviewed, was 21.3%.
Three-month mortality in subgroups of patients
In two studies () survival curves have been presented for patients with stage III and stage IV disease. We calculated that ‘early death burden’ was worse in stage IV disease (mean values in stage III and stage IV patients were 10% and 19% respectively) Citation[22], Citation[30].
Table III. Three-month mortality depending on stage or Karnofsky performance status (KPS) of patients with advanced pancreatic cancer as presented in four studies.
In another two studies survival curves have been presented for good and worse performance status patients Citation[28], Citation[29]. From the data in the papers we calculated that the mean ‘early death burden’ was for patients with a Karnofsky performance status score of 90–100 and 60–80, 15% and 33.5% respectively ()
Reported causes of patients mortality in the first 3 months
We scrutinised the papers for the mention of cause of death for patients who die early in these studies.
In only two studies the cause of 26 early deaths is reported (). Oettle et al. in their study of gemcitabine versus gemcitabine/pemetrexed mention that six patients died from progressive cancer prior to starting chemotherapy Citation[20]. Also Herrmann et al. at the 2005 annual meeting of ASCO, reported 2-month mortality in patients with Karnofsky performance status score of 60–80%. Twenty participants died early and 15 of those died from progressive cancer, three from infections and two from vascular thromboembolic events (myocardial infarction and pulmonary embolism) Citation[29].
Table IV. Causes of death in 26 patients who died within 3 months from their inclusion in the studies.
Cause of death in patients who died while on chemotherapy
In most of these studies reviewed the median duration of treatment was around 3 months, therefore we made the assumption that many of the deaths reported as having happened during treatment could be seen as proportionately reflecting events in the ‘early death burden’ period.
We found data regarding deaths while on chemotherapy in seven studies. In the study of Bramhall et al. testing marimastat and in the study of Bramhall et al. examining the efficacy of the combination gemcitabine/marimastat, all deaths that happened during treatment (although exact number not available) the authors attributed them to progressive cancer Citation[14], Citation[17].
Deaths during treatment from further five studies are shown in . Burris et al., Burch et al., Berlin et al., Oettle et al. and Poplin et al. reported in total 14 deaths. It seems that most deaths were due to infections (neutropenic or not) (5 deaths) and ‘fatal complications of cancer’ (4 deaths) Citation[7], Citation[13], Citation[16], Citation[20], Citation[30].
Table V. Causes of death in 14 patients who died while on treatment (likely to be in the first 3 months).
The authors of three further studies (Maisey et al., Louvet at al., Reni et al.), report that they had no toxic deaths during treatment but do not elaborate further on the cause of death of their patients during treatment.
Causes of death overall in patients who died the first 3 months and during chemotherapy (assuming they also died the first 3 months).
Overall, and given the above postulations, we have a cause of death reported for only 40 of the 1 447 premature deaths. From this reported group progressive disease seems to be the most frequently stated cause of death (52.5%). Other less frequent causes of death reported were infections (20%), complications directly related to cancer not further specified (10%), vascular thromboembolic events (myocardial infarction and pulmonary embolism) (5%), and renal failure that developed while patients receiving chemotherapy (5%).
Discussion
We have found that 23.3% (almost 1 in 4) of patients with advanced pancreatic cancer in recent phase III studies receiving chemotherapy or chemotherapy-based treatment were dead within 3 months from the day of inclusion in these studies. A specific cause of death was reported for only 2.8% of these deaths precluding any evidence-based conclusions for the cause of ‘early death burden’ in advanced pancreatic cancer. However some assumptions can be made.
First of all it seems to us that overall the conventionally viewed toxic deaths due to chemotherapy (neutropenic sepsis, haemolytic uremic syndrome, pulmonary fibrosis and thrombocytopenic bleeding) are likely to represent a very small percentage of the ‘early death burden’. Excluding fatal neutropenic sepsis and acute respiratory distress syndrome, which could also be related to an infection, we found only four toxic deaths from the chemotherapy (renal failure, diabetic ketoacidosis and respiratory arrest). Authors of other studies have reported that they had no toxic deaths and in the vast majority of studies there is no mention about deaths due to chemotherapy. Given the ever-increasing Good Clinical Practice-related regulatory oversight of these trials over the last few years it is unlikely that excess treatment toxicity, especially leading to death, would not have been stringently reported.
Certainly pancreatic cancer patients can be at risk of severe infections mostly biliary sepsis as a consequence of obstruction or due to the fact that many of those might have a biliary stent in situ. However, we believe that at least in the first 3 months when all patients are on active treatment, before first imaging assessment, if any fatal infections did occur they would have been reported within the context of a serious adverse event, as these infections are likely to still be viewed as potential complications of treatment. So that the eight deaths reported due to infection represent a very small percentage of the 1 447 deaths of the ‘early death burden’.
Should we therefore be comfortable with the deduction that almost a quarter of patients that are fit to enter trials of chemotherapy will have died of ‘progressive disease’ within 12 weeks of entry? Advanced pancreatic cancer patients certainly start dying from the minute the trial-treatment commences. Assuming a proportionate distribution it works out as 1.8% per week on trial. We tend to glibly assume that pancreatic cancer is an aggressive disease and biological evidence from in vitro work tends to be cited Citation[31]. What do we mean by aggressive in the clinical sense? If we measure aggressiveness as an ability of this cancer to metastasise widely and kill the host from metastases (e.g. small cell lung cancer, melanoma, sarcoma) then we can demonstrate that in pancreatic cancer lung metastases are not common, bone metastases even less common Citation[32], and brain metastases are the material of case reports Citation[33]. If we measure it as clinical chemoresistance then quite remarkably the same agent (5-fluorouracil) that has provided us with a modest survival advantage in the adjuvant setting for colorectal cancer Citation[34] has done so for pancreatic cancer as well Citation[35].
There is no doubt that progressive disease will be in due course the main cause of death in advanced pancreatic cancer patients mostly through intraperitoneal and liver-related complications but to assume that almost a quarter of the trial-eligible patients with normal to minimally abnormal liver function tests, normal renal function, able to eat and drink and performance status as measured on Karnofsky scale of equal or better than 60% (usual trial-entry criteria apply) will suddenly obstruct or get liver failure or intractable peritoneal carcinomatosis just to fulfil the diagnosis of having died of ‘progressive disease’ is difficult to visualize. Moreover, this assumption is contradictory to the persistently replicated findings of a multitude of autopsy trials/series which actually draw attention to what we think is the most likely explanation of ‘early death burden’; namely the spectre of the vascular thrombotic events. The sheer volume of documented events in multiple studies likely, in our view, to explain a proportional ‘stealth’ mortality.
In the study of Moore et al., who reported cause of death for all participants, ‘progressive cancer’ represented 96% of deaths Citation[18]. What does ‘progressive cancer’ mean in this context especially if in one arm of the study 43% of patients have died before 12 weeks? In a small but insightful study from 1984 in patients with bypassed locally advanced pancreatic cancer treated with helium ion irradiation with or without 5-fluorouracil chemotherapy, efforts were made to obtain a post-mortem at death Citation[32]. This was achieved in 22 patients. All had ‘progressive disease’ at autopsy, 16 with liver metastases, seven with peritoneal metastases and most patients with multiple metastases [including lymph nodes, stomach, duodenum, colon, lung (5), bone (1), adrenal (1), skin(1)] but the cause of death was a pulmonary embolus in ten (45%) of these patients (complicated with evidence of infection in seven). Liver failure and gross ascites was documented only in three cases as the cause of death. No patients had gastrointestinal tract obstruction as cause of death and incidentally five of 22 patients (23%) died within an ‘early death burden’ context (i.e. 2 months after concluding treatment) three of which (60%) from pulmonary emboli.
Evidence of the burden of vascular thromboembolic events in advanced pancreatic cancer stretches back to 1938 Citation[36] and comprehensively reviewed by Khorana et al. Citation[37] so we will limit ourselves only to the citation of a most recent extensive autopsy series by Ögren et al. from Sweden who specifically examined the presence of venous thromboembolism in patients with various types of cancer, including 441 patients with pancreatic cancer. They found that 42% of the pancreatic cancer patients had pulmonary embolism and in at least 14% of patients it was the cause of death Citation[38].
We therefore think that the concept of what constitutes ‘death from progressive disease’ in pancreatic cancer conceals within it a significant burden of mortality due to vascular thromboembolic events and this is deeply disturbing especially when related to fit trial patients that do not reach their first assessment milestone. This is specifically of concern since there is some evidence that chemotherapy per-se and possibly some of the newer anti-angiogenic agents that may become useful in advanced pancreatic cancer do carry a risk of exacerbating the thrombophilic state. How much chemotherapy contributes to thrombosis-related early death cannot be gauged through the methodological approach of our review but a recent epidemiological study by Blom et al. has attempted to quantify this risk and has determined that distant metastases raise the risk of vascular thromboembolic events by 1.9 fold (which may relate to the higher ‘early death burden’ we document in this setting in ), but chemotherapy had a notable 4.8 fold increase in the risk of venous thromboembolism Citation[39]. This statistic gives serious impetus to the need to include vascular thromboembolic events as potential chemotherapy-related causes of ‘toxic death’, specifically in advanced pancreatic cancer, but also to the need to consider anti-thrombotic prophylaxis in patients undergoing chemotherapy.
Some evidence that vascular thromboembolic events represent the major cause of the excessive 3-month mortality can be obtained from some recently published studies of thromboprophylaxis in pancreatic cancer patients undergoing chemotherapy. Nakchabandi et al. presented the results of a retrospective study in 180 patients with locally advanced or metastatic pancreatic cancer. Approximately two thirds of the patients, in addition to the standard regional or systematic chemotherapy, received a fixed low dose of warfarin (1.25 mg daily) without monitoring the INR. With the warfarin the median survival was increased and the ‘early death burden’ dropped from 60 to 21% Citation[40]. Icli et al. a few months ago presented the results of a non-randomised study where half of the 69 patients with advanced pancreatic cancer received prophylaxis with the low molecular weight heparin nadroparine calcium in addition to the chemotherapy doublet gemcitabine/cisplatin. The 3-month mortality as calculated from the survival curves provided was 10% in the arm receiving nadroparin and 36% in the arm receiving only chemotherapy Citation[41]. We recently presented the interim analysis of a randomised IIb trial in patients with advanced pancreatic cancer randomizing to either gemcitabine single agent or gemcitabine and dalteraprin at 200 u/kg for 12 weeks. We found a reduction in vascular thromboembolic events from 30 to 0% and a reduction in ‘early death burden’ from 15 to 4% Citation[42]. These early data from these three trials taken together seem to indicate that a staggering 67–75% of the ‘early death burden’ may be due to thromboembolism.
Finally there is also some interesting information that can be gleaned from a phase III trial. The MALT study that randomised ‘all-comer’ cancer patients to 6 weeks nadroparine and standard of care versus standard of care alone. Survival data given in the paper for the 6-week time-point show that mortality was more than halved in the nadroparine arm (from 10 to 4%) Citation[43]. Although this is not strictly the 12 week endpoint we have attached to ‘early death burden’ it gives us some more information in a prospective sense and also suggests that this may not be solely an advanced pancreatic cancer related problem. There are at least two other malignancies with severe thrombosis related problems, non-small cell lung cancer and stomach cancer Citation[44].
The final point we would like to raise is that of the unknown natural history of the ‘silent’ embolus both that of pulmonary embolism Citation[45] but also of the splanchnic veins. These diagnoses are becoming more common with the more powerful multi-slice computer tomography (CT) scanners that we are using and in our experience seem to be a not uncommon finding during advanced pancreatic cancer staging.
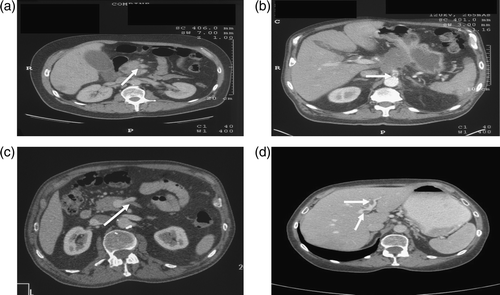
Closing we present in (a–d) some of our own experience from staging CT scans of pancreatic cancer patients demonstrating ‘silent’ splanchnic thrombi. The first (a) image is from a 59-year-old patient who presented with jaundice and was found to have cancer of the head of the pancreas with liver metastases. The initial CT scan revealed a superior mesenteric vein thrombus. The second (b) image shows a celiac axis thrombosis in a 70-year-old patient complaining of weight loss and chronic epigastric pain and found to have locally advanced cancer of the body of the pancreas. Image (c) is from a 72-year-old patient presenting with anemia and early satiety. He was found to have a poorly differentiated adenocarcinoma of the body of the pancreas invading into stomach with a lymph node mass at the celiac axis and an the initial CT scan revealed inferior mesenteric vein thrombus. Image (d) is from a 65-year-old patient with locally advanced pancreatic cancer compromising the portovenous confluence and two thrombi in the left portal venous system (arrows) with no other evidence of distal (main) portal vein thrombosis.
Figure 1 a–d. “Silent” thrombosis of the splanchnic vessels in advanced pancreatic cancer patients. a: Thrombus in the superior mesenteric vein (arrow), b: thrombus in the origin of the celiac axis (arrow), c: thrombus in the inferior mesenteric vein (arrow), d: thrombosis in the left portal vein system (arrows).
Conclusions
‘Early death burden’ in advanced pancreatic cancer seems to be a real phenomenon and only part of a continuum of a serious thromboembolic tendency that afflicts these patients. Oncologists need to be aware of the fact that a large proportion of their advanced pancreatic cancer patients are at risk of early death and that vascular thromboembolic events may be contributing significantly to this risk some of which may be treatment related. Modern trials in advanced pancreatic cancer need to start documenting carefully the elements that constitute ‘death from progressive disease’ in this cancer and possibly view venous thromboembolic events as a treatment related complication. The position of thromboprophylaxis needs to be investigated vis a vis best anticoagulant, optimal dose, and stage of disease setting to try and decrease stealth mortality that probably relates to the whole life of a pancreatic cancer patient but is nowhere felt more acutely than when being treated with chemotherapy in expectation of a response.
Acknowledgements
We would like to thank Dr Ged Avery, and Dr Alex Paddon for their assistance in the preparation of the figure with the CT scan images. Also Andrew Dove for his significant contribution in the literature search.
References
- Cancer Research UK. UK pancreatic cancer mortality statistics (Assessed 14 April 2007 at http://info.cancerresearchuk.org./cancerstats/types/pancreas/mortality/).
- Pancreatic cancer action network. Pancreatic cancer facts for 2007 (Assessed 11 April 2007, at http://pancan.org/About/pancreaticCancerStats.html).
- Cardenes HR, Chiorean EG, DeWitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: Current therapeutic approach. Oncologist 2006; 11: 612–23
- Yeo CJ, Yeo TP, Hruban RH, Kern SE, Iacobuzio-Donahue CA, Maitra A, . Cancer of the pancreas. Cancer. Principles and practice of Oncologyth, VT De Vita, Jr, S Hellman, SA Rosenberg, et al. Lippincott Williams and Willkins, Philadelphia 2005; 945–986
- Agnieszka M,Hill M,Maraveyas A,Wasan H,Lofts F. Phase I/II study to investigate the use of gemcitabine in combination with raltitrexed in locally advanced or metastatic pancreatic adenocarcinoma. Internet J Oncol 2006; 3.
- Michael A, Hill M, Maraveyas A, Dalgleish A, Lofts F. 13-cis-retinoic acid in combination with gemcitabine in the treatment of locally advanced and metastatic pancreatic cancer–report of a pilot phase II study. Clin Oncol (R Coll Radiol) 2007; 19: 150–3
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: A randomized trial. J Clin Oncol 1997; 15: 2403–13
- Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R, et al. A randomised trial comparing 5-FU with 5-FU plus cisplatin in advanced pancreatic carcinoma. Ann Oncol 2002; 13: 1185–91
- Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: A prospective randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 2002; 94: 902–10
- Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vernenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004; 22: 1430–8
- Shapiro J, Marshall J, Karasek P, Fige A, Oettle Ha, Couture F, et al. G17DT + gemcitabine [Gem] versus placebo +Gem in untreated subjects with locally advanced, recurrent or metastatic adenocarcinoma of the pancreas: Results of a randomized, double-blind, multinational, multicenter study. J Clin Oncol 2005 ASCO Annual Meeting Proceedings 2005; 23(16S):4012.
- Wright JA, Osterlee J, Fekete S, Lee Y, Young AH. A phase III trial of virulizin plus gemcitabine vs. gemcitabine alone in advanced pancreatic cancer: Results of subgroup analysis. J Clin Oncol 2006 Annual Meeting Proceedings 2006; 24(18S):4116.
- Burch PA, Block M, Schroeder G, Kugler JW, Sargent DJ, Braich TA, et al. Phase III evaluation of octreotide versus chemotherapy with 5-fluorouracil or 5-fluorouracil plus leucovorin in advanced exocrine pancreatic cancer: A north central cancer treatment group. Clin Cancer Res 2000; 6: 3486–92
- Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JAC. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: A randomized trial. J Clin Oncol 2001; 19: 3447–55
- Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, et al. Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-fu) with PVI 5-fu plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 2002; 14: 3130–6
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller D, Benson AB, 3rd. Phase III study of gemcitabine in combination with flurouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002; 20: 3270–5
- Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JAC. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 2002; 87: 161–7
- Moore MJ, Hamm J, Dancey J, Eisenberg PD, Fields A, Hagan K, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21: 3296–302
- Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey M, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004; 22: 3776–83
- Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 2005; 16: 1639–45
- Reni M, Cordio S, Milandri C, Passoni P, Bonetto E, Oliani C, et al. Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: A randomised controlled multicentre phase III trial. Lancet Oncol 2005; 6: 369–76
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005; 23: 3509–16
- Heinneman V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006; 24: 3946–52
- Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, et al. A multicenter phase III study comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 2006; 95: 587–92
- Abou-Alfa GK, Letourneau R, Harker G, Modiano M, Hurwitz H, Tchekmedyian NS, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006; 24: 4441–7
- Chevreton P, Friess H, Andras C, Salek T, Geddes C, Bodoky G et al . Phase III results of exatecan (DX-8951f) versus gemcitabine (Gem) in chemotherapy naive patients with advanced pancreatic cancer (APC). J Clin Oncol, 2004 ASCO Annual Meeting Proce;22(14S):4005.
- Moore MJ Goldstein D Hamm J Figer A Hecht J Gallinger S et al . Erlotinib plus gemcitabine compared to gemcitabine alone, in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. J Clin Oncol, 2005 ASCO Annual Meeting Proc;23(16S):1.
- Riess H, Helm A, Niedergethmann M, Schmidt-Wolf I, Moik M, Hammer C et al . A randomised, prospective, multicenter, phase III trial of gemcitabine, 5-fluorouracil (5-FU), folinic acid vs. gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol, 2005 ASCO Annual Meeting Proc;23(16S):4009.
- Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Saletti P, Bajetta E , et al . Gemcitabine (G) plus capecitabine (C) versus G alone in locally advanced or metastatic pancreatic cancer. A randomized phase III study of the Swiss Group for Clinical Cancer Research (SAKK) and the Central European Cooperative Oncology Group (CECOG). J Clin Oncol, 2005 ASCO Annual Meeting Proc;23(16S):4010.
- Poplin E Levy DE Berlin J Rothenberg M, Cella D, Mitchell E et al . Phase III trial of gemcitabine (30-minute infusion) versus gemcitabine (fixed-dose-rate infusion[FDR]) versus gemcitabine + oxaliplatin(GEMOX) in patients with advanced pancreatic cancer (E6201). J Clin Oncol, 2006 ASCO Annual Meeting Proc;24(18S):LBA4004.
- Neoptolemos JP, Cunningham D, Friess H, Bassi C, Stocken DD, Tait DM, et al. Adjuvant therapy in pancreatic cancer: Historical and current perspectives. Ann Oncol 2003; 14: 675–92
- Woodruff KH, Castro TJR, Quivey JM, Saunders WM, Chen GT, Lyman JT, et al. Post-mortem examination of 22 pancreatic carcinoma patients treated with helium ion irradiation. Cancer 1984; 53: 420–5
- Park K, Kim M, Park S, Lee KJ. Nervous system involvement by pancreatic cancer. Neuro-Oncol 2003; 63: 313–6
- Goyle S, Maraveyas A. Chemotherapy for colorectal cancer. Dig Surg 2005; 22: 401–14
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001; 358: 1576–85
- Sproul EE. Carcinoma and venous thrombosis: The frequency of association of carcinoma in the body or tail of the pancreas with multiple venous thrombosis. Am J Cancer 1938; 34: 566–85
- Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol 2004; 5: 655–63
- Ögren M, Bergqvist D, Wahlanden K, Eriksson H, Sternby NH. Trousseau's syndrome–what is the evidence? A population-based autopsy study. Thromb Haemost 2006; 95: 541–5
- Blom JW, Osanto S, Rosendall FR. High risk of venous thrombosis in patients with pancreatic cancer: A cohort study of 202 patients. Eur J Cancer 2006; 42: 410–4
- Nakchbandi W, Muller H, Singer MV, Lohr M, Nakchandi IA. Effects of low-dose warfarin and regional chemotherapy on survival in patients with pancreatic carcinoma. Scand J Gastroenterol 2006; 41: 1095–104
- Icli F, Akbulut H, Utkan G, Yalcin B, Dincol D, Isikdogan A, et al. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J Surg Oncol 2007; 95: 507–12
- Maraveyas A Holmes M Lofts F Garadi KK Gardiner E Sgouros J Chemoanticoagulation versus chemotherapy in advanced pancreatic cancer (APC): Results of the interim analysis of the FRAGEM trial. J Clin Oncol 2007, ASCO Annual Meeting Proceedings 2007;25(18S):4583.
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 2005; 23: 2130–5
- Hillen HF. Thrombosis in cancer patients. Ann Oncol 2000; 11(Suppl3)273–6
- Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. Am J Roentgenol 2005; 184: 264–7