Abstract
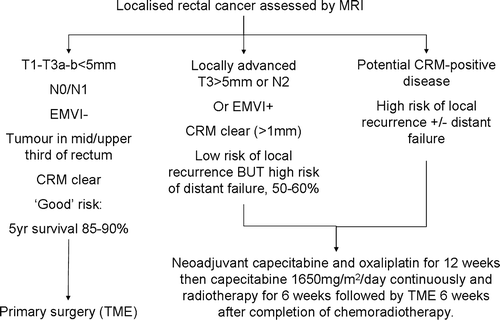
Detailed preoperative staging using high resolution magnetic resonance imaging (MRI) enables the selection of patients that require preoperative therapy for tumour regression. This information can be used to instigate neoadjuvant therapy in those patients with poor prognostic features prior to disturbing the tumour bed and potentially disseminating disease. The design of trials incorporating MR assessment of prognostic factors prior to therapy has been found to be of value in assessing treatment modalities and outcomes that are targeted to these preoperative prognostic subgroups and in providing a quantifiable assessment of the efficacy of particular chemoradiation treatment protocols by comparing pre-treatment MR staging with post therapy histology assessment. At present, we are focused on achieving clear surgical margins of excision (CRM) to avoid local recurrence. We recommend that all patients with rectal cancer should undergo pre-operative MRI staging. Of these, about half will have good prognosis features (T1–T3b, N0, EMVI negative, CRM clear) and may safely undergo primary total mesorectal excision. Of the remainder, those with threatened or involved margins will certainly benefit from pre-operative chemoradiotherapy with the aim of downstaging to permit safe surgical excision. In the future, our ability to recognise features predicting distant failure, such as extramural vascular invasion (EMVI) may be used to stratify patients for neo-adjuvant systemic chemotherapy in an effort to prevent distant relapse. The optimal pre-operative treatment regimes for these patients (radiotherapy alone, systemic chemotherapy alone or combination chemo-radiotherapy) is the subject of current and future trials.
Staging is the method of summarising the anatomical extent of a malignant tumour, thereby communicating information regarding prognosis. Historically, the development of cancer staging has its origins in the descriptive studies of Dukes in the field of rectal cancer Citation[1]. The pathological staging of tumours into distinct prognostic groups relies exclusively on static morphologic features seen in the resected surgical specimen. Prognostic factors that predict for risk of disease failure from either local recurrence, distant metastases or both were identified from careful analysis of clinico-pathological data Citation[1–4]. However, it was not possible to improve patient outcomes from that predicted by histological staging because at the time that many of these landmark studies were being carried out, surgery remained the only treatment for rectal cancer.
Over time, the efficacy and safety of other anti-cancer treatments, including chemotherapy and radiotherapy has been evaluated and these have become available as adjuvant treatments for patients with rectal cancer. Overall survival was shown to be significantly improved in those patients with pathologically demonstrated lymph node metastases if they were given postoperative 5-FU chemotherapy Citation[5]. When radiotherapy was given postoperatively to all patients with rectal cancer a significant reduction in local recurrence rates was observed Citation[6], and when radiotherapy was combined with chemotherapy, local control was better Citation[7]. For the first time, the clinical outcome could be improved from the prognosis offered by post-operative pathological staging. However, clinical decision-making still relied on the histopathological staging, to determine which patients might gain the most benefit from receiving these potentially harmful treatments. Any pre-operative imaging performed was essentially carried out to diagnose or exclude the presence of visible metastases (usually hepatic or pulmonary). Patients found to have synchronous metastastic disease could be diverted into either a non-surgical palliative treatment plan, or referred for specialist assessment for metastasectomy.
The importance of pre-operative staging for rectal cancer
The identification of prognostic factors by standard pathological assessment is of great importance, but for the postoperative patient, the primary therapy (i.e. surgery) has already been delivered. Useful prognostic information about likelihood of survival may be obtained, but it may be too late to influence survival substantially since the opportunity to downstage and produce tumour regression prior to surgery has been lost. With advances in oncology and widening of therapeutic options, primary surgery is no longer the only treatment, or necessarily the most appropriate treatment for rectal cancer. Adjuvant radiotherapy reduces rates of local recurrence Citation[8], with the greatest benefits seen in patients receiving radiotherapy pre-operatively. The Swedish Rectal Cancer Trial demonstrated a survival benefit in patients undergoing pre-operative radiotherapy + surgery versus surgery alone Citation[9], although this observation has not been repeated in other studies. The potential advantages of pre-operative versus post-operative treatments means that a technique which identifies poor prognostic factors preoperatively could be applied to select patients for neoadjuvant therapy prior to disturbing the tumour bed and potentially disseminating the disease. Furthermore, the intensity of preoperative therapy may be modified according to prognosis. Efficacy in terms of local control and reduced toxicity are both improved when chemoradiation is used before rather than after surgery Citation[10].
MRI staging of rectal cancer
An optimal pre-operative staging technique would accurately identify prognostic factors preoperatively, enabling the selection of patients into appropriate treatment groups. High-resolution MRI has been shown to be superior to clinical examination (digital rectal examination), computed tomography, and endoluminal ultrasound (EUS) for staging of rectal tumours. Tumours of the distal sigmoid, rectosigmoid, and upper rectum can all be staged accurately using MRI Citation[11]
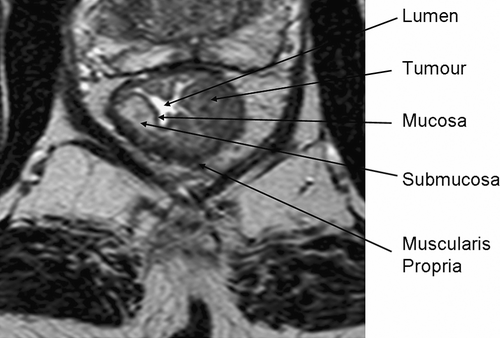
Since the late 1990s, sophisticated multiple element coil arrays have been developed. These coils were designed to have the advantages of the surface coil by obtaining higher signal but with greater coverage and improved homogeneity. This was achieved by combining surface coils in to an array of four (or more) coils, which are connected to amplifiers and multiple receiver channels. The signals undergo mathematical post processing to produce a single image from the information received by each coil. Thin (3 mm) sections with the pelvic phased-array coil give similar in-plane resolution as that obtained with the endorectal coil, but with much wider coverage. Using a high-resolution protocol Citation[12] (), MRI can not only distinguish tumour from rectal wall (), but also consistently depict the mesorectal fascia Citation[13] () and anatomical structures that relate to the optimal surgical technique for rectal cancer Citation[14]. In addition, we use a standardised reporting proforma ().
Figure 1. Saggital scout image using standard high resolution protocol: 3 mm slice interval, 16–18 cm FOV, 4–6 NSA, 256×256 matrix, TR >3,000, TE 80–100, ETL 16. In plane resolution 0.6×0.6 mm.
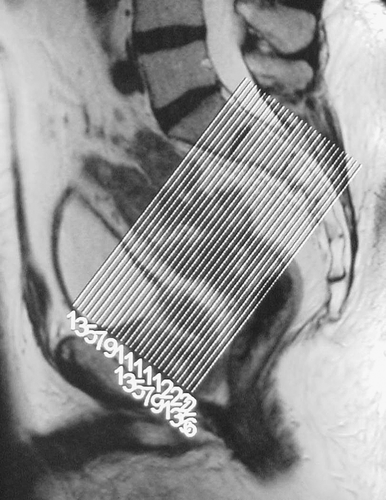
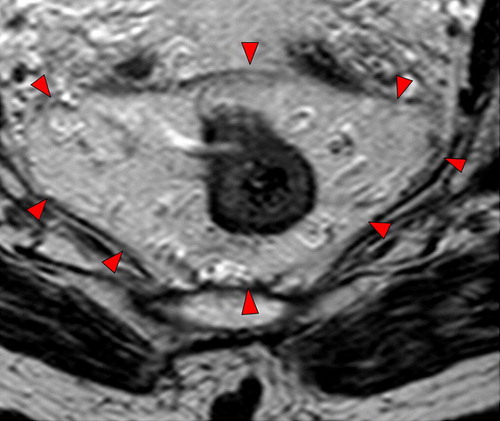
Figure 3. High resolution T2-weighted axial MRI through rectal tumour showing the fascia propria of the mesorectum (red arrowheads) which corresponds to the surgical circumferential resection margin.
We will now discuss the main prognostic factors that have been identified by clinico-pathological studies, and see how MRI can be used to stage each of these factors pre-operatively.
Pre-operative identification of prognostic factors in rectal cancer
The TNM system
The TNM system uses the principle that the anatomical extent of the disease may be based on the size and extent of the primary tumour (T), the absence or extent of regional lymph node metastasis (N) and the presence or absence of distant metastasis (M). The greater the extent of the cancer in each of the TNM categories, the higher the numerical modifier (ranging between 0 and up to 4). TNM is versatile in that it allows for both clinical and pathological assessment of stage. The various combinations of T-stage, N-stage and M-stage are clustered into stage groupings (Stage I to IV) based on prognosis. The TNM system for staging cancer of the colon and rectum was originally devised to correspond directly with the original Dukes system Citation[1]: Stage I, Dukes A; Stage II, Dukes B; Stage III, Dukes C. Stage IV corresponds to the presence of distant metastases. The prognostic accuracy of the TNM system is ensured by its periodic revision, taking into account data from outcome studies. This process of continuous improvement, together with its comprehensive set of definitions and rules of application and its multidisciplinary design are cited as key advantages over other staging systems Citation[15]. Consequently, this system is the standard for colorectal cancer staging, recommended by international bodies including the Royal College of Pathologists of England and the College of American Pathologists. The latest (sixth) revision was published in 2002 Citation[16].
Within each T stage there is a heterogeneous survival range and there has been much interest in identifying poor prognostic groups within each stage. Both T1 and T2 tumours have high 5 year survival but the widest range in survival is demonstrated in patients with T3 tumours, which make up 80% of rectal tumours seen in clinical practice. A number of authors have shown a relationship between survival and the depth of extramural spread that is independent of other prognostic factors including the circumferential margin status Citation[17], Citation[18]. With respect to depth of tumour invasion, the sixth edition of the TNM classification suggests an optional expansion of the classification of pT3 tumours according to measured extramural invasion (<1mm; 1–5mm; >5–15mm; >15mm). This represents the most precise subdivision yet to be proposed and reflects the accumulated body of evidence, which can be traced right back to 1932 and Dukes’ original observations.
1. MRI prediction of extramural spread/T-Stage
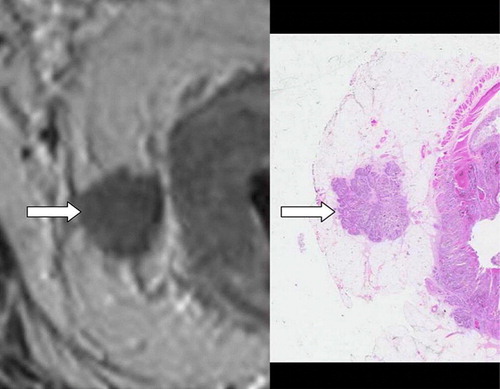
Thin-slice high resolution MRI can be used to accurately measure the depth of extramural spread. A prospective series of 28 patients undergoing pre-operative MRI using a four-element surface coil was the first study to shows good correlation with histology Citation[13]. In that study 21/24 (88%) of patients with tumour infiltrating 5 mm or over in to perirectal fat were correctly identified. In most cases, extramural depth, as measured using MRI, showed direct agreement with corresponding histopathological measurements. Cases of discordance between MRI and histology measurement of extramural depth occurred in ulcerating tumours that produced erosion of the muscularis propria. This was because of difficulty in identifying the muscularis propria and thus measuring distance from the outer muscle coat when an eroding ulcer was present. We were also able to show good agreement between pathology and MRI assessment of T-stage in a larger prospective study of 98 patients (weighted κ = 0.67) Citation[19].
The Sharp Memorial Hospital in San Diego, Californian published a retrospective evaluation of their use of MRI to stage both rectal and colonic cancers in a series of 48 patients Citation[20]. They reported 84% agreement between pre-operative MR and final pathological T-stage in 19 rectal tumours and overall TNM stage was correctly predicted in 95%. In another series of 40 patients, T-staging was correctly predicted in only 20 (12 under-staged, 8 over-staged) although a clear learning curve was evident as MRI prediction became more accurate over the course of the prospective study Citation[21]. A recent study from Tokyo demonstrated good correlation between MRI and depth of invasion, with at least 82% accuracy for T-stage prediction (κ = 0.82) Citation[22].
The largest study to date looking at the accuracy of MRI staging of rectal cancer has been the MERCURY study, a multicentre prospective trial which recruited 679 consecutive patients with rectal cancer in 11 centres in Britain, Germany, Norway and Sweden Citation[23]. After exclusions for missing data, direct comparison of the extramural depth of invasion measured by MRI and histology was made in 295 (94.9%) of 311 patients undergoing primary surgery. The mean difference between MRI and histopathology was −0.046 mm (SD = 3.85 mm, 95% confidence interval −0.487 to 0.395 mm). Thus MRI has been shown to be equivalent to within 0.5 mm for prediction of local tumour spread.
2. Lymph node involvement
Preoperative assessment of lymph node status in rectal cancer is of importance for a number of reasons. Firstly, the number of involved nodes has an influence on prognosis Citation[3], Citation[5] Second, the presence of tumour-containing lymph nodes close to the mesorectal fascia (which forms the conventional surgical circumferential resection margin) increases the risk of recurrence Citation[24] unless the standard excision plane is altered. Nodes lying outside the mesorectal fascia may require extended lymphadenectomy in order to achieve clearance of tumour Citation[25], Citation[26]; those that remain unresected may be responsible for local recurrence despite apparently clear surgical resection margins Citation[27]. The use of neoadjuvant preoperative therapy may be influenced by the presence of nodes containing tumor close to the potential resection plane. Finally, the ability to determine reliably node-negative status preoperatively could result in less aggressive surgery and preoperative therapy in some patients.
A major challenge for any cross-sectional imaging modality lies in the ability to predict lymph node status prior to surgery, not least because involved nodes may contain only microscopic tumour foci, and therefore be normal-sized. Inability to predict nodal status in patients with rectal cancer is viewed as an important limitation of pre-operative staging techniques. An analysis of 437 lymph nodes harvested from 42 Total Mesorectal Excision specimens, with careful comparison of the axial pre-operative MRI images and the matched transversely-sectioned histopathology specimens showed that the diameters of benign and malignant nodes were similar Citation[28]. The finding that no particular size cut-off is useful in predicting nodal status is supported by a histological survey of over 12 000 lymph nodes in rectal cancer Citation[29] that showed considerable size overlap between normal or reactive nodes and those containing metastases.
Studies using endoluminal ultrasound found that the internal texture of an imaged node may correlate better with the presence of metastasis than nodal size Citation[30–32]. We applied these observations to high resolution MR evaluation of lymph node status, and demonstrated that intranodal signal heterogeneity (i.e. a mixed signal) was a highly specific discriminant. When lymph nodes were defined as suspicious based on having an irregular border, or mixed signal intensity, greater accuracy was achieved, with sensitivity of 85% and specificity of 97%. In another study, we demonstrated 85% accuracy (κ = 0.68) comparing MRI prediction of lymph node status with histology in a prospective series of 98 patients Citation[19]. In both series, prediction of lymph node status showed good reproducibility between observers and was independent of, and greatly superior to, lymph node size.
Fifteen to 42% of small (<5 mm) mesorectal lymph nodes in rectal cancer patients may contain metastases Citation[28], Citation[29], Citation[33]. In the Cardiff study, 23% of all the nodes harvested from resection specimens were missed by MRI, but these were all <3 mm and only two of the 102 contained metastases Citation[28]. On the other hand, this technique did correctly identify many nodes measuring 2–5 mm, and correctly predicted the presence of metastases in some based on irregular contour. Whilst its power to resolve such small nodes is clearly suboptimal, it would seem that MR evaluation of nodes using border/signal criteria will result in understaging of few patients. Nevertheless, the inability to detect microscopic metastases in all lymph nodes suggests that a negative MR examination should not be used to select patients for local excision surgery.
Work in our department using ultra-small particles of iron oxide (USPIO) has shown promising results in terms of identifying very small (>1 mm) foci of tumour within mesorectal nodes Citation[34]. Malignant nodes (confirmed by histological correlation) have been shown to have a characteristic appearance on T2-weighted high-resolution MRI following administration of USPIO which is different to the appearance of reactive or non-malignant nodes.
MRI cannot necessarily distinguish between a lymph node replaced by tumour and a separate extramural tumour deposit in the mesorectum. Nevertheless since patients with discontinuous tumour deposits have a much worse prognosis Citation[35], Citation[36], it is another advantage of the technique that such deposits can be detected pre-operatively.
3. Tumour involvement of the Circumferential Resection Margin
The association between local recurrence after surgery for rectal cancer and tumour involvement of the lateral (now called ‘Circumferential’) resection margin was first demonstrated in 1986 by Professor Quirke's group in Leeds Citation[37]. They examined operative specimens from 52 patients with rectal cancer, and found that in 14 (27%) tumour had spread to the lateral margin of resection, and that 12 of these patients went on to develop pelvic recurrence. They concluded that incomplete surgical excision was therefore the cause for most local recurrences in rectal cancer. Two larger studies by the Leeds group revealed that not only did CRM involvement by tumour increase the risk of local recurrence, but that it also predicted poor survival. Patients with CRM involvement (defined as tumour within 1 mm of the resection margin Citation[38]) were reported to have 3.5 times the risk of local recurrence and double the risk of death Citation[24], Citation[39].
Total mesorectal excision
The risk of local recurrence after surgical resection of rectal cancer is much lower when the mesorectum is excised intact Citation[40], Citation[41]. The mesorectum is a distinct anatomical unit comprising the rectum, perirectal fat, blood vessels, nerves and lymphatic vessels. It is surrounded by a layer of fibroareolar tissue called the mesorectal fascia. The detailed anatomy of the mesorectal fascia was described only relatively recently by Bissett et al. Citation[42], who dissected the fascia propria off the mesorectum and showed that the fascia was a continuous structure that encircled the rectum and mesorectum, fusing with the peritoneum as it reflected off the rectum. At the level of the anorectal junction, the mesorectum thins out.
Total mesorectal excision (TME) is performed by sharp dissection along the plane that separates the visceral from the parietal layers of the perirectal pelvic fascia thus enabling the radical removal of the rectum within its surrounding mesorectumv Citation[43]. The principle rationale for this method of surgery is the total removal of rectum and its draining lymph nodes en bloc, preserving the anal sphincter and autonomic nerves. Most rectal tumours are confined to within the mesorectal ‘packet’, and the results of Professor Heald Citation[44] and others Citation[45] lend support to Quirke's observation that incomplete surgical excision is largely responsible for local recurrences. Initial concerns that the results of TME surgery were not reproducible Citation[46] were refuted by subsequent studies. For example Dixon et al. demonstrated a 5 year survival of 64% after TME surgery Citation[47]. Similar results have been corroborated at other centres with local recurrence rates falling from 30% with conventional surgery to under 10% following TME Citation[48], Citation[49].
The widespread acceptance of TME surgery as the gold standard operative procedure for patients with rectal cancer promises to be one of the single most important factors in reducing local recurrence Citation[41], Citation[45], Citation[50], Citation[51]. Nevertheless, the CRM may still be positive if the tumour extends up to or through the mesorectal fascia. A national audit of rectal cancers in Norway confirmed that even after TME surgery, 9% of patients had a positive CRM, and the local recurrence rate in this group was 22% compared to 5% for those with negative CRM Citation[52]. The importance of CRM status as a prognostic factor is not therefore diminished by TME, although the incidence of CRM positivity is much lower when TME is performed.
MRI assessment of potential resection margins (CRM)
Curative sphincter-preserving surgical resection of rectal cancer is dependent on the complete removal of all macroscopic and microscopic tumour in the pelvis. The tumour must be clear of adjacent anatomical structures, the mesorectal fascia and the sphincter complex. The MRI appearances of the anatomical structures relevant to TME, including the peritoneal reflection, Denonvilliers’ fascia, the pelvic nerve plexuses, mesorectal fascia and mesorectum itself have all been described Citation[14]. The mesorectal fascia represents the potential CRM in patients undergoing TME surgery. Bissett et al. conclusively demonstrated by using markers that the mesorectal fascial plane seen with MRI Citation[42] corresponds to the fascia propria encasing the mesorectum and excised by TME. MRI can therefore be used to assess the distance from the tumour edge to the potential circumferential margin and thereby predict the final CRM status in patients undergoing TME surgery.
Studies have used different cut-off values of the measured distance to the mesorectal fascia to predict CRM status. The authors of one study concluded that CRM status could be predicted with a high degree of accuracy and consistency Citation[53] when a cut-off of 5 mm MRI measured distance to the CRM was used. Other authors have been able to use more precise MRI measurements to predict CRM status. Our own prospective study involving 98 patients undergoing pre-operative MRI staging, predicted the CRM status to be positive when tumour was identified by MRI within 1 mm of the mesorectal fascia. Comparison with histology showed very good (92%, κ = 0.81) agreement Citation[19] (). In a different British study, high-resolution pre-operative MRI correctly predicted the CRM status (the cut-off value was not reported) in 39/40 cases Citation[21].
A recently published meta-analysis which compared MRI against histology after total mesorectal excision included data from nine studies (which did not include MERCURY) involving 529 patients. This confirmed that high-resolution MRI accurately predicts tumour involvement of the CRM with a sensitivity and specificity of 94% and 85% respectively Citation[54]. The multi-centre MERCURY study itself demonstrated accurate prediction of CRM status throughout the 11 participating hospitals. Of 408 consecutive patients undergoing surgery after MRI staging, 354 had clear CRM on histology. Three hundred and twenty seven of three hundred and fifty four of these were correctly predicted by the pre-operative MRI giving a specificity of 92.4%. The negative (clear margin) predictive value was 94% Citation[55]. Overall accuracy for prediction of a clear margin was higher for the 311 patients undergoing primary surgery (91%) than for those 97 who completed pre-operative chemo-radiotherapy or long-course radiotherapy (77%).
Abdomino-Perineal excision rates have declined in specialist colorectal centres as evidence accumulates for the oncological safety of sphincter-saving procedures Citation[43].
4. Extramural Vascular Invasion (EMVI)
The landmark pathological studies into vascular invasion were published in the early 1980s by Professor Ian Talbot Citation[56–58], and the body of evidence in the literature suggesting that vascular invasion is of prognostic significance continues to grow. Amongst these histological studies, the rate of detection of vascular invasion is variable with incidences ranging from 17 to 70% reported Citation[56], Citation[59–65]. Even in patients undergoing careful radical excision of the rectum and mesorectum, venous invasion remains an important independent prognostic factor Citation[40], Citation[66] EMVI is associated with higher risk of local recurrence Citation[64], distant metastases Citation[29], Citation[62], Citation[67–69] and death Citation[4], Citation[62], Citation[64], Citation[70–74].
The typical appearance on MRI, which is the only imaging modality that has been shown to demonstrate extramural vascular invasion in rectal cancer Citation[19] is that of discrete serpiginous or tubular projections of intermediate signal intensity into perirectal fat, following the course of a visible perirectal vessel (usually a vein). We have previously reported that extramural vascular invasion was correctly identified on 15 of 18 patients with rectal cancer Citation[19]. More recently, we have devised an MRI-EMVI grading score to evaluate the presence or absence of radiological features indicative of EMVI (). In a recent study carried out in our own unit Citation[75] we found that the proportion of patients with MRI-detected EMVI in 94 rectal and rectosigmoid cancers undergoing primary surgery was 26%, which was similar to the histologically-proven proportion (28%). The sensitivity and specificity of MRI for detecting EMVI in this series was 62% and 88% respectively. Some patients with microscopic vascular invasion could not be resolved on MRI, while others with very obvious EMVI on the pre-operative images had false-negative histopathology due to obliteration of normal venous architecture which makes it difficult for the pathologist to recognise that a tumour deposit lies within the course of a vessel, something which may be more readily appreciated on serial MR images ().
Figure 6. A tumour nodule is seen apparently extending laterally from the right side of the primary tumour. There is no histological evidence that this nodule is associated with any vascular structure on this slide. Serial ascending axial MRI slices through the same tumour suggest that the nodule lies within a tubular structure running parallel to the bowel wall, and the upper-most image shows signal void indicating that this is likely to be a vein.
Table I. Summary of MRI-EMVI scoring system.
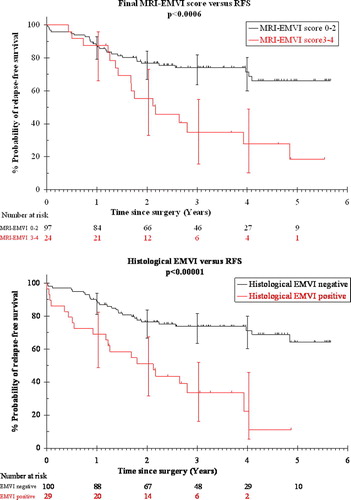
In the same paper, we were able to show that MRI-detected EMVI is associated with poor clinical outcome. Despite MDT-directed pre-operative and post-operative therapy, the presence of EMVI on a pre-operative MRI scan was associated with a four-fold higher risk of distant metastasis (52% versus 12%), and a reduction in relapse-free survival at 3 years to only 35% versus 74% for patients with no EMVI ().
Figure 7. The presence of EMVI is associated with a significant reduction in relapse-free survival whether it is identified pre-operatively (upper graph) or histologically (lower graph). (Fig 7: Reproduced with permission. N. J. Smith, Y. Barbachano, A. R. Norman, R. I. Swift, A. M. Abulafi, G. Brown. Permission is granted by John Wiley & Sons Ltd on behalf of the BJSS Ltd Copyright # 2007 British Journal of Surgery Society Ltd).
5. Peritoneal involvement
Locally-advanced upper rectal tumours may perforate through the peritoneum. In one prospective study of 412 colon cancers, local peritoneal involvement was found to be an independent risk factor for intraperitoneal recurrence after surgery. Similarly in rectal cancer, peritoneal involvement predicts for local recurrence.
The typical appearance seen on rectal MRI is one of nodular extension of intermediate signal intensity through the fine low-signal-intensity peritoneal reflection at or above the level of its attachment to the anterior surface of the rectum: this is best demonstrated on axial high resolution images. The Cardiff series included 11 patients with pT4 tumours, nine of whom had histological evidence of perforation with tumour cells seen on the peritoneal surface. This was correctly identified in 7/9 cases. In two other cases, MRI suggested nodular tumour infiltration through the peritoneum, and while histology confirmed that the tumour was close to the peritoneal surface, there was actually no perforation Citation[19]. Although cases of peritoneal perforation were undoubtedly identified using preoperative MRI, many cases will be missed by MRI due to failure to resolve microscopic infiltration of peritoneal lined clefts. The accuracy of MRI in correctly identifying peritoneal involvement at this site is therefore less reliable than detection of other prognostic factors Citation[76]. However, knowledge of the relationship of tumour to the peritoneal reflection anteriorly should prompt a careful search for subtle peritoneal infiltration.
Pre-operative therapy and the multi-disciplinary team approach to the management of patients with rectal cancer
We recommend a selective approach to pre-operative therapy in rectal cancer, with selection based on MRI-identified features occurring in the context of the multi-disciplinary team (MDT). This team of surgeons, radiologists, oncologists, pathologists and associated clinical nurse specialists is now established as the optimum approach to the management of patients with rectal cancer. We have shown that rectal cancer patients discussed in the MDT meeting pre-operatively had lower rates of histological CRM involvement (2% versus 26%), and that patients with irresectable disease could be identified and offered alternative treatment Citation[77]. Detailed pre-operative MDT discussion based on MRI staging is therefore essential to the aim of achieving resection with clear margins.
The benefits of a selective approach using MRI based selection criteria are self evident. Approximately 40–50% of patients can be treated successfully with primary surgery without significant risk of local recurrence or systemic failure. Of the remainder, potentially dramatic improvements may be achieved through the use of intensive and targeted preoperative therapy aimed not only at reducing the size of the primary tumour and rendering potentially irresectable tumour resectable with tumour free circumferential margins but also to enable patients at high risk of systemic failure to benefit from intensive combined modality therapy aimed at eliminating micrometastatic disease (). The characterisation of a patient's tumour as either “good” (no adverse features), “bad” (features suggesting increased risk of systemic relapse, although surgical margins clear and therefore resectable) or “ugly” (surgical margins involved) may suggest three possible pre-operative therapeutic strategies. However, the optimal strategy for each is still under investigation Citation[78].
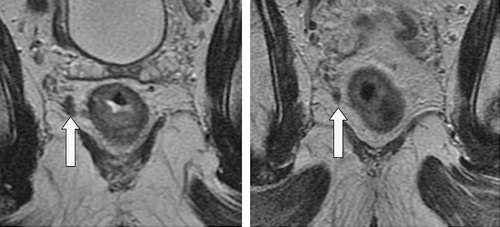
Figure 8. Extramural venous invasion lying close to the mesorectal resection margin. The image on the right shows the same level after chemoradiotherapy with some downstaging evident.
We routinely use high spatial resolution MRI to select patients with poor risk tumours for intensive pre-operative therapy. This approach has been tested prospectively in a phase II trial () which showed an objective tumour response to systemic chemotherapy followed by combination chemo-radiotherapy in 88% of patients. Sixty-six of 67 (99%) patients with originally-threatened or involved margins who underwent surgery had R0 resections Citation[79].
Conclusions
Detailed preoperative staging using high resolution MRI enables the selection of patients that require preoperative therapy for tumour regression. This information can be used to instigate neoadjuvant therapy in those patients with poor prognostic features prior to disturbing the tumour bed and potentially disseminating disease. An MRI-directed multidisciplinary team approach to the identification of patients at high risk for local and/or distant failure is recommended. The optimal pre-operative treatment regimes for these patients (radiotherapy alone, systemic chemotherapy alone or combination chemo-radiotherapy) is the subject of current and future trials.
Acknowledgements
With thanks to Mrs Barbara Mason for her administrative support in the preparation of this paper.
References
- Dukes CE. The classification of cancer of the rectum. J Path Bact 1932; 35: 323–32
- Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954; 139: 846–52
- Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet 1987; 1(8545)1303–6
- Harrison JC, Dean PJ, el-Zeky F, Vander Zwaag R. From Dukes through Jass: Pathological prognostic indicators in rectal cancer [see comments]. Human Pathol 1994; 25: 498–505
- Moertel CG, Fleming TR, MacDonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med 1995; 122: 321–6
- Anonymous. Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party [see comments]. Lancet 1996;348(9042):1610–4.
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–15
- Anonymous. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358(9290):1291–304.
- Anonymous. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial [see comments] [published erratum appears in N Engl J Med 1997 May 22;336(21):1539]. New Engl J Med 1997;336:980–7.
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Burton S, Brown G, Daniels I, Norman AR, Swift I, Abulafi AM, et al. MRI identified prognostic features of tumors in distal sigmoid, rectosigmoid, and upper rectum: Treatment with radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys 2006; 65: 445–51
- Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol 2005; 78(927)245–51
- Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: Thinsection MR imaging for staging in 28 patients. Radiology 1999; 211: 215–22
- Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol 2004; 182: 431–9
- Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 2004; 54: 295–308
- Sobin LH Wittekind C TNM Classification of Malignant Tumours (UICC). 6th ed. 2002.
- Cawthorn SJ, Parums DV, Gibbs NM, A'Hern RP, Caffarey SM, Broughton CI, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer [see comments]. Lancet 1990; 335(8697)1055–9
- Willett CG. Technical advances in the treatment of patients with rectal cancer [editorial; comment]. Inter J Radiat Oncol Biol Phys 1999;45:1107–8.
- Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 2003; 90: 355–64
- Low RN, McCue M, Barone R, Saleh F, Song T. MR staging of primary colorectal carcinoma: Comparison with surgical and histopathologic findings. Abdom Imaging 2003; 28: 784–93
- Branagan G, Chave H, Fuller C, McGee S, Finnis D. Can magnetic resonance imaging predict circumferential margins and TNM stage in rectal cancer?. Dis Colon Rectum 2004; 47: 1317–22
- Akasu T, Iinuma G, Fujita T, Muramatsu Y, Tateishi U, Miyakawa K, et al. Thin-section MRI with a phased-array coil for preoperative evaluation of pelvic anatomy and tumor extent in patients with rectal cancer. AJR Am J Roentgenol 2005; 184: 531–8
- MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY study. Radiology 2007;243:132–9.
- Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994; 344(8924)707–11
- Suzuki K, Muto T, Sawada T. Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today 1995; 25: 795–801
- Billingham RP. Extended lymphadenectomy for rectal cancer: Cure vs quality of life. Int Surg 1994; 79: 11–22
- Moreira LF, Kenmotsu M, Gochi A, Tanaka N, Orita K. Lymphovascular and neural invasion in low-lying rectal carcinoma. Cancer Detect Prevent 1999; 23: 123–8
- Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of highspatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: 371–7
- Gunther K, Dworak O, Remke S, Pfluger R, Merkel S, Hohenberger W, et al. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res 2002; 103: 68–78
- Hildebrandt U, Klein T, Feifel G, Schwarz HP, Koch B, Schmitt RM. Endosonography of pararectal lymph nodes. In vitro and in vivo evaluation. Dis Colon Rectum 1990; 33: 863–8
- Hildebrandt U, Feifel G. Importance of endoscopic ultrasonography staging for treatment of rectal cancer. Gastrointest Endosc Clin N Am 1995; 5: 843–9
- Hulsmans FH, Bosma A, Mulder PJ, Reeders JW, Tytgat GN. Perirectal lymph nodes in rectal cancer: in vitro correlation of sonographic parameters and histopathologic findings. Radiology 1992; 184: 553–60
- Schnall MD, Furth EE, Rosato EF, Kressel HY. Rectal tumor stage: Correlation of endorectal MR imaging and pathologic findings [see comments]. Radiology 1994; 190: 709–14
- Koh DM, Brown G, Temple L, Raja A, Toomey P, Bett N, et al. Rectal cancer: Mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings–initial observations. Radiology 2004; 231: 91–9
- Ueno H, Mochizuki H, Tamakuma S. Prognostic significance of extranodal microscopic foci discontinuous with primary lesion in rectal cancer. Dis Colon Rectum 1998; 41: 55–61
- Singh AK, Myerson RJ, Birnbaum EH, Fleshman JW, Kodner IJ, Lockett MA, et al. Outcome of patients with rectal adenocarcinoma and localized pelvic non- nodal metastatic foci. Dis Colon Rectum 2000; 43: 1217–21
- Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986; 2(8514)996–9
- Hall NR, Finan PJ, al-Jaberi T, Tsang CS, Brown SR, Dixon MF, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence?. Dis Colon Rectum 1998; 41: 979–83
- Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002; 235: 449–57
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1(8496)1479–82
- Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002; 89: 1142–9
- Bissett IP, Fernando CC, Hough DM, Cowan BR, Chau KY, Young AA, et al. Identification of the fascia propria by magnetic resonance imaging and its relevance to preoperative assessment of rectal cancer. Dis Colon Rectum 2001; 44: 259–65
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence?. Br J Surg 1982; 69: 613–6
- Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; 133: 894–9
- Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg 1996; 83: 1112–5
- Hermanek P. Impact of surgeon's technique on outcome after treatment of rectal carcinoma. Dis Colon Rectum 1999; 42: 559–62
- Dixon AR, Maxwell WA, Holmes JT. Carcinoma of the rectum: A 10-year experience. Br J Surg 1991; 78: 308–11
- Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995; 181: 335–46
- McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis 1995; 10: 126–32
- Nesbakken A, Nygaard K, Westerheim O, Mala T, Lunde OC. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol 2002; 28: 126–34
- Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000; 356(9224)93–6
- Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 2002; 89: 327–34
- Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001; 357(9255)497–504
- Purkayastha S, Tekkis PP, Athanasiou T, Tilney HS, Darzi AW, Heriot AG. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis 2007; 9: 402–11
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006;333(7572):779.
- Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins in cancer of the rectum. Br J Surg 1980; 67: 439–42
- Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: Method of detection, histological features and significance. Histopathology 1981; 5: 141–63
- Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg 1981; 141: 15–7
- Tsuchiya A, Ando Y, Kikuchi Y, Kanazawa M, Sato H, Abe R. Venous invasion as a prognostic factor in colorectal cancer. Surg Today 1995; 25: 950–3
- Lui KK, Enjoji M, Inokuchi K. Venous permeation of colorectal carcinoma. Jap J Surg 1980; 10: 284–9
- Sunderland D. The significance of vein invasion by cancer of the rectum and sigmoid: A microscopic study of 210 cases. Cancer 1949; 2: 429–37
- Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer 1988; 61: 1018–23
- Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer 1988; 61: 1417–24
- Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer 1983; 52: 1317–29
- Knudsen JB, Nilsson T, Sprechler M, Johansen A, Christensen N. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum 1983; 26: 613–7
- Bokey EL, Ojerskog B, Chapuis P, Dent O, Newland RC, Sinclair G. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: Role of total anatomical dissection. Br J Surg 1999; 86: 1164–70
- Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, et al. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer 1996; 78: 2313–7
- Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. A prospective clinicopathologic study of venous invasion in colorectal cancer. Am J Surg 1991; 162: 216–22
- Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum 1991; 34: 798–804
- Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg 1985; 72: 698–702
- Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 1984; 2(8405)733–6
- Horn A, Dahl O, Morild I. The role of venous and neural invasion on survival in rectal adenocarcinoma. Dis Colon Rectum 1990; 33: 598–601
- Mulcahy HE, Toner M, Patchett SE, Daly L, O'Donoghue DP. Identifying stage B colorectal cancer patients at high risk of tumor recurrence and death. Dis Colon Rectum 1997; 40: 326–31
- Newland R, Dent O, Lyttle M, Chapuis P, Bokey E. Pathological determinants of survival associated with colorectal cancer with lymph node metastases. Cancer 1994; 73: 2076–82
- Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2007 (in press).
- Brown G, Daniels IR. Preoperative staging of rectal cancer: The MERCURY research project. Recent Results Cancer Res 2005; 165: 58–74
- Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D. MRI directed multidisciplinary team preoperative treatment strategy: The way to eliminate positive circumferential margins?. Br J Cancer 2006; 94: 351–7
- Glimelius B. Rectal cancer irradiation. Long course, short course or something else?. Acta Oncol 2006; 45: 1013–7
- Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poorrisk rectal cancer. J Clin Oncol 2006; 24: 668–74