Abstract
Introduction. Converging epidemiological evidence based on studies of different designs in a variety of populations and settings show that cancer survival tends to be poorer in low compared to high socioeconomic groups. In an extension of an earlier register-based study, we examined the influence of socioeconomic factors on long-term survival in women with a first diagnosis of invasive breast cancer in 1993 in Sweden, a country with a policy of providing equal access to health care to all at nominal cost within a National Health Care System. Material and methods. The study was based on data set generated by record linkages between the Swedish Cancer Register, Census databases and the Cause of Death Register. Four different categorical variables were used as indicators of socioeconomic standing. Cox proportional hazard regression models were used to estimate the effects of socioeconomic status on risk of death. Results. Of 4 645 eligible women with breast cancer, 1 016 had died from breast cancer at the end of follow-up on December 31, 2003. After adjustment for tumour size and age at diagnosis, risk of death was 19% lower among women belonging to a household of high compared to low socioeconomic status (HR high versus low 0.81; 95% CI: 0.67–0.97). Discussion. These findings indicate that social inequalities in breast cancer survival persist at least up to ten years after an initial diagnosis. While social gradients detected shortly after diagnosis may mainly reflect an influence of socioeconomic differences in overall health status and frailty, differentials persisting beyond five years rather point to a long-term influence of disparities in management of both primary tumours and recurrences. Further studies are needed to explore whether the present findings reflect amendable inequalities in access to state-of- the-art treatment. For all calendar periods, observed survival in the most privileged groups sets the goal for what is achievable for all breast cancer patients.
Converging epidemiological evidence based on studies of different designs in a variety of populations and settings show that cancer survival tends to be poorer in low compared to high socioeconomic groups Citation[1–5]. These gradients appear to be most pronounced in cancers with good prognoses, such as breast cancer for which tumour stage at diagnosis is an important prognostic factor Citation[4], Citation[6].
We have previously found evidence of socioeconomic inequalities in 5-year survival in breast cancer in Sweden Citation[7], a country with a policy of providing equal access to health care to all at nominal cost within a National Health Care System. Outreach mammography screening programs were established in Sweden in the late 1980s, while the management of breast cancer has been guided by regional consensus guidelines.
Using an expanded and updated version of a data-set used in our earlier study, the aim of the present investigation was to examine whether the previously observed social differences in survival remain detectable long-term. While five-year survival is a commonly used measure of cancer outcome, assessment of ten-year survival has become increasingly relevant as prognosis continues to improve over time for many malignancies. Also, extended follow-up allows more precise estimates of risk of death, based on a larger number of events. The majority of studies to date exploring social disparities in breast cancer survival have been based on assessments of outcome five years following diagnosis.
Material and methods
The materials and methods have previously been described in detail Citation[7]. In short, our study was based on a data set generated by record linkages between the Swedish Cancer Register, five Regional Cancer Registers, the 1980, 1985 and 1990 Census databases, the Fertility Register, the Migration Register, and the Cause of Death Register. Record linkages were made possible by an individually unique National Registration Number (NRN) assigned to each resident in Sweden.
Patients and their follow-up
A total of 5 853 breast cancers (in 5 649 women) diagnosed from January 1 to December 31, 1993 were identified in the Swedish Cancer Register. We restricted the analyses to women with no diagnoses of invasive cancers prior to 1993, and to women with adenocarcinoma of the breast to minimize the influence of differences in tumour biology. Additional diagnoses recorded more than one month after the first diagnosis in 1993 were considered as new tumours (n = 171, of which 85 were breast cancers). Cases first diagnosed at autopsy (n = 10) were included in the analyses as having zero survival. The final study population consisted of 4 645 women. Patients were followed up regarding emigration and death through record linkages to the Migration Register and the Cause of Death Register until December 31, 2003. Following a manual review of death certificates retrieved from the Cause of Death Register, one case initially registered as having died from another cause was recoded as dead from breast cancer.
The study was approved by the Research Ethics Committee at Karolinska Institute.
Prognostic factors
Age at diagnosis was studied both in continuous and in categorical (<50, 50–59, 60–69, 70–79, >80) form. Information on tumour characteristics at diagnosis (tumour size and nodal involvement classified according to the TNM system, and oestrogen receptor status) was obtained from five of the six Regional Cancer Registers, covering 80.4% of the female population in Sweden.
Sociodemographic factors
For the purpose of the present study, four different categorical variables were used as indicators of socioeconomic standing.
A socioeconomic index variable, a commonly used indicator for socioeconomic status (SES) in Sweden based on occupational group Citation[8], was stratified into six levels; unskilled blue-collar workers, skilled blue collar workers, low, intermediate and high level white collar workers and self-employed (including entrepreneurs and farmers). SES was also categorised into two levels; low (including blue collar workers and low level white collar workers) and high (intermediate and high level white collar workers and self-employed). Women classified as not gainfully employed included unemployed, housewives and pensioners. Information on SES was available for the woman as well as for the household, in which case level of SES was based on the person in the household who had the highest SES. If information on SES was missing or the woman was not gainfully employed in the 1990 Census, information from the Census databases on 1985 or 1980 was used, if providing useful additional data.
Educational level was classified into low (mandatory school < 9 years), medium (high school 10–12 years) and high (college and university, >12 years). Individual disposable income and household disposable income were stratified into quartiles. Household income includes earnings from the index person and all other household members. Home ownership was divided into renting and owning.
Additional sociodemographic factors considered in the analyses included cohabitation (categorized as no or yes, where yes included married women), parity (nulliparous and parous), total number of people living in the household (including the woman) categorized into two groups (1, >1), country of origin (Sweden, Nordic countries and non-Nordic countries), geographical region of residence at time of diagnosis according to the coverage of six Regional Cancer Registers (Göteborg, Linköping, Lund, Norrland, Uppsala and Stockholm), and access to an outreach mammography screening program as yes or no.
Statistical methods
Time to event techniques were used: the Kaplan-Meier method was used to estimate the cause-specific survival distribution of time from diagnosis of breast cancer to death. Women contributed to person-time until they died, emigrated or were alive at the end of follow-up. Log-rank based tests were used to assess the crude effect of socioeconomic status on breast cancer survival and Cox proportional hazards regression models were fitted to the survival data to estimate the effect of socioeconomic status (both women and household), while adjusting for the possible confounding effects of relevant demographic and clinical variables. Parameter estimates and 95% confidence intervals (CI) were obtained by maximizing the partial log likelihood. Model fitting and residual analyses were based on graphical and statistical tests, using Schoenfeld and cumulative Martingales residuals. The statistical software STATA 9 was used to carry out the analyses.
Results
During the study period there was a total of 1 937 deaths, of which 1 016 were due to breast cancer, corresponding to an overall 10 year cause-specific survival proportion of 78.1%.
Tumour stage, lymph node involvement and oestrogen receptor status were factors that all had strong prognostic impact. Women with small size tumours (0–20 mm) at diagnosis had a ten-year cause-specific survival proportion of 87.7% compared to 43.1% in women with large tumours (>50 mm) ().
Table I. Characteristics of 4 645 women diagnosed with invasive breast cancer (BC) in 1993. Distribution of demographic and cancer-related variables by survival status on December 31, 2003.
Women aged 50–59 years at diagnosis had a survival proportion of 80.8% ten years later, while the corresponding estimate in women 80 years or older was 74.6%. In the youngest age group (<50 years), the cause specific survival proportion was 76.1%.
Kaplan-Meier survival curves for a maximum of ten years of follow-up stratified by SES group are shown in . In the group of low SES women, the cause-specific survival at ten years was 78%, compared to 82% in women of high SES. Similarly, the survival proportions were lower in the groups of low household SES, low income and no home ownership, compared to more privileged groups (). Educational level and a more detailed classification of socioeconomic status based on occupation were also associated with survival. The best survival was observed in women with middle level education, while no difference was seen between patients with high and low levels of education. For socioeconomic status, the poorest survival proportion was observed in the group not employed (76%) which was slightly lower than the estimate for the unskilled blue collar workers (77.3%).
Figure 1. Kaplan-Meier stratified survival curves for a maximum of 10 years of follow-up. Women with a first diagnosis of invasive breast cancer in 1993. Follow-up to December 31, 2003.
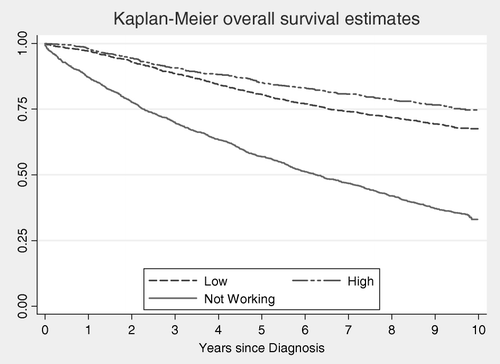
In the second part of the analysis, risk of death due to breast cancer was computed and adjusted for age and tumour size at diagnosis. A similar pattern was seen for almost all socioeconomic indicators following adjustment for age, with hazard ratios (HR) being lower among women with high compared to low socioeconomic status, albeit not always statistically significant (). However, following adjustment also for tumour size, the only socioeconomic indicator that remained significantly associated with risk of death was household SES (HR = 0.81; 95% CI: 0.67–0.97). In the group of high SES women, a significant gradient became more pronounced following exclusion of the self-employed (HR = 0.78; 95% CI: 0.61–0.98) (data not shown).
Table II. Social factors and tumour characteristics and all-cause risk of death in women diagnosed with breast cancer in 1993. Hazard ratios for death, estimates adjusted for age at diagnosis* and for age in combination with tumour size at diagnosis.**
Compared to small tumours, the hazard ratio for large tumours was 7.00 (95% CI 5.34–9.17). There was evidence of a better prognosis for women living in the Linköping region compared to women residing in other administrative regions. Women living in areas with outreach mammography were at significantly lower risk of death from breast cancer (HR = 0.70; 95% CI: 0.50–0.97).
The socioeconomic difference in survival was most pronounced in women younger than 50 years at diagnosis (HR = 0.57; 95% CI: 0.53–0.87) (). In women older than 80 years at diagnosis, the pattern was reversed with some evidence of a higher risk of death among women in high SES households.
Table III. Socioeconomic standing (woman and household) and hazard ratios (high vs low) for all cause death by age group at diagnosis, and adjusted for tumour size.
In a final step, all variables that were significant (p-value <0.20) in univariate analyses were considered as potential candidates in a multivariate proportional hazards model in which age in continuous form was kept independently of its significance. The models were stratified both by tumour stage and lymph node status due to their highly non-proportional hazards. Thus, the final model presented in shows the hazard ratio estimate of the socioeconomic standing adjusted by parity and cohabitation, confirming the protective effect of high versus low social status (HR = 0.73; 95% CI: 0.56–0.96).
Table IV. Social factors and tumour characteristics and the risk of death (all causes). Multivariate proportional hazard model.
In a separate step, all analyses were repeated with all-cause mortality as an outcome. With this approach, the overall pattern was similar showing a social gradient in survival (data not shown).
Discussion
Our finding of a social gradient in survival remaining up to ten years after diagnosis confirms the pattern observed in our earlier study based on fewer deaths during a five-year follow-up Citation[7]. Not only did the main pattern remain long-term, but the results for subgroups were also almost identical. Irrespective of measure used to represent social status, the survival tended to be worse in lower socioeconomic groups, although the differences did not always reach formal statistical significance. The observed disparities, however, were for the most part rather modest which may reflect that social inequalities remain relatively small in the Swedish society. A study comparing breast cancer survival between socioeconomic groups in Canada and USA showed a poorer prognosis for low SES women with breast cancer in the USA compared to low SES breast cancer patients in Canada Citation[9], a finding which may reflect the same phenomenon.
Survival differed between age groups. Older women generally had a worse prognosis compared to younger age groups, possibly reflecting an influence of a greater comorbidity burden and age-biased management resulting in undertreatment of elderly patients Citation[10]. However, one of the poorest survival rates were found in premenopausal patients (<50 years) which may partly be explained by more aggressive tumour characteristics and possibly also detrimental influences of recent pregnancies in young women Citation[11]. The social gradient also appeared to be strongest in the youngest age group, which could reflect that the management of breast cancer varies more in this numerically smaller and clinically more heterogeneous group for whom treatment guidelines may be less strictly adhered to. Also, at the time of diagnosis of the patients in the present study, only a small proportion of women below the age of 50 were covered by an outreach mammography screening program. However, we found no difference in the distribution of tumour size at diagnosis between young and old breast cancer patients.
Possible underlying causes for reported socioeconomic differences in cancer survival can be attributed to factors related to the tumour, the patient, and the management of the disease Citation[5], Citation[12].
The tumour: Early detection and tumour characteristics
In our study, the socioeconomic variation in survival remained following adjustment for tumour characteristics which corroborates findings in some earlier studies Citation[7], Citation[13], Citation[14], indicating that observed gradients cannot be explained solely by differences in timing and stage at diagnosis Citation[15], Citation[16]. In 1993, the year of diagnosis for all breast cancer cases in the present study, 95% of the female population 50 years or older was covered by screening programs. While we previously have been unable to detect pronounced socioeconomic differences in attendance in outreach mammography screening in central Sweden Citation[17], other investigators have found evidence of socioeconomic gradients in utilization in an urban area in southern Sweden Citation[18]. There is some evidence suggesting that tumour biology may differ between socioeconomic groups with negative oestrogen receptor status being more common among deprived women Citation[19]. In our study, we did not find any such differences. In one study, histopathological tumour type could explain only 3% of the elevated risk of death among low socioeconomic groups Citation[16], while other investigators have found no associations between socioeconomic factors and tumour size, nodal status, oestrogen receptor status and histological grade Citation[20].
The patient: Comorbidity, life-style, psychosocial factors, awareness and compliance
Burden of comorbidity may contribute to observed social gradients in cancer survival. Findings in an American study showed that a high comorbidity score was associated with higher overall mortality rates in breast cancer patients Citation[21]. However, that study did not examine breast cancer specific mortality and the groups were compared based on ethnicity, and not socioeconomic standing. In a Dutch study, other chronic health conditions were more prevalent at the time of breast cancer diagnosis among low and medium SES women compared to high SES women Citation[22]. In the present study, we found evidence of an all-cause higher risk of death in low socioeconomic groups across indicators of socioeconomic standing which may reflect burden of concomitant disease, which in turn could influence both management choices and how well the patient accepts, responds to and tolerates breast cancer therapy.
Lifestyle factors such as smoking and overweight, which are known to be over-represented in groups of low socioeconomic status Citation[23], Citation[24], may negatively influence breast cancer prognosis Citation[21], Citation[25] not only directly, but also indirectly by inducing comorbidity that could affect treatment and tumour progression. There is also some epidemiological evidence indicating that psychosocial factors could influence progression and survival in breast cancer Citation[26], Citation[27]. Results from a recent animal study showed that exposure to chronic behavioural stress resulted in higher levels of cathecolamines, greater tumour burden and more invasive growth of ovarian carcinoma cells in a mouse model. Tumours in stressed animals showed increased vascularisation, pointing towards the effects of stress on tumour growth being mediated by angiogenic processes Citation[28]. It remains unclear, however, if and to what extent psychosocial factors correlate with socioeconomic status or if they are mainly influenced by individual circumstances. More recently, increasing attention has been given to disparities in the awareness of the disease such as health care seeking behaviours and knowledge about breast cancer and its treatment which could influence patient compliance Citation[29].
Management of the disease
Socioeconomic differences in management and access to state-of-the-art treatment may play a central role for inequalities in survival. In a US setting, it has been shown that affluent women are more likely to receive both breast conservative surgery and endocrine therapy compared to more deprived women Citation[19]. However, in at least one earlier report, differences in survival outcome between socioeconomic groups remained after adjustment for treatment Citation[15]. In another study, stage-specific management did not differ between women of different SES groups Citation[30]. Findings from a recent US-based study showed that black women were more likely to stop treatment prematurely and that early termination was associated with poorer survival. However, the association between SES and ethnicity is complex Citation[21].
Strengths of the present study include the prospective, population-based design and the ability to asses the influence of a series of different measures of socioeconomic status which were determined on the individual or household level. The influence of changing treatment practices over time was minimized by focusing on breast cancer cases diagnosed during one calendar year. All tumours had been classified histopathologically, and only adenocarcinomas were included in the analyses. Non-random misclassification was not an issue, since information on outcome was collected independently of information on tumour characteristics and socioeconomic indicators. In a validation of breast cancer recorded as an underlying cause of death in the Swedish Cause of Death Register, less than 2% of deaths were judged to be in disagreement with the conclusions of an end point committee that reviewed medical information case by case Citation[31]. In this setting, with high quality data available on cause of death, cause-specific survival proportion estimates provide an acceptable alternative to computing survival proportions (relative survival) using social class-specific death rates to estimate expected survival Citation[32].
A weakness of our study was the absence of data on treatment and the inclusion of women classified as not employed, a heterogeneous group including pensioners, unemployed and housewives which both had the highest overall mortality, and the highest cause-specific mortality. The majority of women in this group were pensioners with 78% of the patients being older than 70 years of age at diagnosis. Also, women with missing data probably represent a selected group and had a low overall survival. Similarly, women classified as self-employed are likely to belong to a heterogeneous group. For that reason analyses were made with and without this group in the high SES category. The relatively large number of missing data on parity and educational level reflects the age distribution, and data on these factors was not available for the oldest birth cohorts. Further, it cannot be excluded that educational level was misclassified in some older women due to changes over time in the Swedish school system.
In conclusion, we found that social inequalities in breast cancer survival persist up to ten years following diagnosis. A socioeconomic gradient detected shortly after diagnosis may mainly reflect adverse influences on the likelihood of survival mediated by socioeconomic differences in overall health status and frailty, while disparities persisting beyond five years rather would point to a long-term influence of suboptimal management and treatment of primary tumours or recurrences. To improve the understanding of the origins of the observed disparities, further studies are needed to explore in detail not only the role of breast cancer management and treatment by social group, but also comorbidity, lifestyle and psychosocial factors. Furthermore, to our knowledge, no study to date has investigated the genetic properties of breast cancer tumours in relation to socioeconomic factors. Social differences in cancer survival are of public health importance since identification of factors amendable to intervention can help prevent unnecessary premature deaths and save human lives. For all calendar periods, observed survival in the most privileged groups sets the goal for what is achievable in all breast cancer patients.
Acknowledgements
We thank the Regional Oncological Centres in Lund, Göteborg, Linköping, Uppsala and Umeå for providing data for this study. This work was supported by a grant from the Swedish Cancer Society (4837 B03, 2003).
References
- Auvinen A, Karjalainen S, Pukkala E. Social class and cancer patient survival in Finland. Am J Epidemiol 1995; 142: 1089–102
- Coleman MP, Babb P, Sloggett A, Quinn M, De Stavola B. Socioeconomic inequalities in cancer survival in England and Wales. Cancer 2001; 91: 208–16
- Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E. Socioeconomic status and cancer survival in Ontario. J Clin Oncol 1997; 15: 1680–9
- Kogevinas M, Porta M. Socoeconomic differences in cancer survival: A review of the evidence. IARC Scientific Publications No 138, M Kogevinas, N Pearce, M Susser, P Boffetta. International Agency for Research on Cancer, Lyon 1997; 177–206
- Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: A review. Ann Oncol 2006; 17: 5–19
- Rosso S, Faggiano F, Zanetti R, Costa G. Social class and cancer survival in Turin, Italy. J Epidemiol Community Health 1997; 51: 30–4
- Lagerlund M, Bellocco R, Karlsson P, Tejler G, Lambe M. Socio-economic factors and breast cancer survival–a population-based cohort study (Sweden). Cancer Causes Control 2005; 16: 419–30
- Swedish Socioeconomic Classification. Reports on statistical coordination, 1982:4 ÖrebroSweden: Statistics Sweden; 1982.
- Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol 1999; 17: 2244–55
- Eaker S, Dickman PW, Bergkvist L, Holmberg L. Differences in management of older women influence breast cancer survival: Results from a population-based database in Sweden. PLoS Med. 2006; 3: e25
- Rosenberg L, Thalib L, Adami HO, Hall P. Childbirth and breast cancer prognosis. Int J Cancer 2004; 111: 772–6
- Auvinen A, Karjalainen S. Possible explanations for social class differences in cancer patient survival. IARC Scientific Publications No 138, M Kogevinas, N Pearce, M Susser, P Boffetta. International Agency for Research on Cancer, Lyon 1997; 377–397
- Karjalainen S, Pukkala E. Social class as a prognostic factor in breast cancer survival. Cancer 1990; 66: 819–26
- Brewster DH, Thomson CS, Hole DJ, Black RJ, Stroner PL, Gillis CR. Relation between socioeconomic status and tumour stage in patients with breast, colorectal, ovarian, and lung cancer: Results from four national, population based studies. BMJ 2001; 322: 830–1
- Schrijvers CT, Mackenbach JP, Lutz JM, Quinn MJ, Coleman MP. Deprivation and survival from breast cancer. Br J Cancer 1995; 72: 738–43
- Kaffashian F, Godward S, Davies T, Solomon L, McCann J, Duffy SW. Socioeconomic effects on breast cancer survival: Proportion attributable to stage and morphology. Br J Cancer 2003; 89: 1693–6
- Lagerlund M, Sparen P, Thurfjell E, Ekbom A, Lambe M. Predictors of non-attendance in a population-based mammography screening programme; socio-demographic factors and aspects of health behaviour. Eur J Cancer Prev 2000; 9: 25–33
- Zackrisson S, Andersson I, Manjer J, Janzon L. Non-attendance in breast cancer screening is associated with unfavourable socio-economic circumstances and advanced carcinoma. Int J Cancer 2004; 108: 754–60
- Thomson CS, Hole DJ, Twelves CJ, Brewster DH, Black RJ. Prognostic factors in women with breast cancer: Distribution by socioeconomic status and effect on differences in survival. J Epidemiol Community Health 2001; 55: 308–15
- Carnon AG, Ssemwogerere A, Lamont DW, Hole DJ, Mallon EA, George WD, et al. Relation between socioeconomic deprivation and pathological prognostic factors in women with breast cancer. BMJ 1994; 309: 1054–7
- Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol 2005; 23: 6639–46
- Schrijvers CT, Coebergh JW, Mackenbach JP. Socioeconomic status and comorbidity among newly diagnosed cancer patients. Cancer 1997; 80: 1482–8
- Lissner L, Johansson SE, Qvist J, Rossner S, Wolk A. Social mapping of the obesity epidemic in Sweden. Int J Obes Relat Metab Disord 2000; 24: 801–5
- Lindstrom M, Ostergren PO. Intermittent and daily smokers: Two different socioeconomic patterns, and diverging influence of social participation. Tob Control 2001; 10: 258–66
- Manjer J, Andersson I, Berglund G, Bondesson L, Garne JP, Janzon L, et al. Survival of women with breast cancer in relation to smoking. Eur J Surg 2000; 166: 852–8
- Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: A population-based cohort study. Lancet 1999; 354: 1331–6
- Watson M, Homewood J, Haviland J, Bliss JM. Influence of psychological response on breast cancer survival: 10-year follow-up of a population-based cohort. Eur J Cancer 2005; 41: 1710–4
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12: 939–44
- Gansler T, Henley SJ, Stein K, Nehl EJ, Smigal C, Slaughter E. Sociodemographic determinants of cancer treatment health literacy. Cancer 2005; 104: 653–60
- Franzini L, Williams AF, Franklin J, Singletary SE, Theriault RL. Effects of race and socioeconomic status on survival of 1,332 black, Hispanic, and white women with breast cancer. Ann Surg Oncol 1997; 4: 111–8
- Nystrom L, Larsson LG, Rutqvist LE, Lindgren A, Lindqvist M, Ryden S, et al. Determination of cause of death among breast cancer cases in the Swedish randomized mammography screening trials. A comparison between official statistics and validation by an endpoint committee. Acta Oncol 1995; 34: 145–52
- Dickman PW, Auvinen A, Voutilainen ET, Hakulinen T. Measuring social class differences in cancer patient survival: is it necessary to control for social class differences in general population mortality? A Finnish population-based study. J Epidemiol Community Health 1998; 52: 727–34