Abstract
Introduction. Taxane/platinum combinations exhibit synergistic cytotoxicity and activity against a broad range of solid tumours. We sought to optimise the regimen as a suitable outpatient palliative treatment for cancer of unknown primary (CUP). Patients and methods. Eligible CUP patients with adenocarcinoma or poorly differentiated carcinoma, performance status of 0-2, adequate organ function and assessable disease were treated with docetaxel 75 mg/m2 and carboplatin at an area under the concentration time-curve (AUC) of 5, both as 30-minute intravenous infusions, every three weeks. Patients with isolated axillary adenopathy, squamous cell cervical or inguinal adenopathy and PSA or germ-cell serum tumour markers were excluded. Results. Forty-seven patients entered the trial, 24 with predominantly nodal disease or non-mucinous peritoneal carcinomatosis (favourable risk) and 23 with visceral metastases (unfavourable risk). A median of 6 cycles of chemotherapy were administered, with relative dose intensities of both drugs >90%. Response rates were 32% (46% in favourable risk, 17% in unfavourable), comparable to the activity of paclitaxel/platinum regimes, though complete remissions were seen only in favourable risk patients. Granulocyte-colony stimulating factor support was used in a third of treatment cycles. Toxicity was mild and manageable, with grade 3–4 neutropenia in 26% of patients, febrile neutropenia in 7% and severe non-hematologic side-effects in less than 8% of patients. No toxic deaths or severe neurotoxicity were seen. Median time to progression (TTP) and overall survival (OS) were 5.5 and 16.2 months respectively. Survival was driven mainly by favourable-risk patients (22.6 months), as those with visceral metastases had a poor median survival of only 5.3 months. Good performance status and low-volume disease predicted for superior outcome, while docetaxel relative dose-intensity was a positive prognosticator only in favourable-risk patients. Conclusions. One-hour docetaxel/carboplatin is a convenient, safe and effective outpatient palliative treatment for CUP patients, providing meaningful survival prolongation only in favourable-risk patients. Insights in the molecular biology of CUP are needed for the development of targeted therapeutic manipulations of malignant resistance and progression.
Cancer of unknown primary (CUP) represents 3–5% of malignancies and encompasses a heterogeneous group of tumours that disseminate early while the primary remains dormant or regresses Citation[1]. Only a minority of patients diagnosed with distinct clinicopathologic subsets of CUP (isolated axillary lymphadenopathy in women, non-mucinous peritoneal carcinomatosis in women, squamous cell cervical lymphadenopathy, and predominantly nodal disease of midline distribution) have reasonable chances for long-term disease control. Unfortunately, the overall outlook is grim as most patients present with high-volume visceral metastases that are resistant to therapy Citation[2]. Moreover, a substantial proportion of the aforementioned favourable prognosis CUP patients succumb to progression of their malignancy, as shown by the mediocre survival of women with peritoneal carcinomatosis (median 15 months) despite reported complete response rates as high as 36% Citation[3]. Accordingly, we are still in dire need of an active antineoplastic treatment for patients with both favourable and unfavourable CUP.
Docetaxel is a mitotic spindle poison which exhibited significant antineoplastic efficacy in several solid tumours, including breast, ovarian, gastrointestinal tract, head/neck and lung cancer. It was shown to retain activity in the presence of p53 mutations and BCL2 overexpression, molecular aberrations commonly encountered in CUP, and its 60-minute infusion schedule offers convenience for administration in the outpatient setting compared to the relative compound, paclitaxel Citation[4]. Carboplatin is a platinum compound that has been effectively used for the management of patients with the most common solid tumours and, in contrast to taxanes, is not a substrate for gp170, the cellular efflux pump that mediates multidrug resistance. The platinum/taxane combination has exhibited synergistic cytotoxicity in malignant cell lines and xenografts and proved effective in the clinical setting against several malignancies Citation[5], Citation[6]. Docetaxel/carboplatin was shown to be less neurotoxic than paclitaxel/carboplatin in ovarian cancer trials Citation[7]. Based on these data, we conducted a phase II trial in order to assess the efficacy and safety of outpatient docetaxel/carboplatin in relatively fit patients with cancer of unknown primary site.
Patients and methods
The Hellenic Cooperative Oncology Group opened accrual to this phase II single-arm study in January 2000 via ten participating centres. Patients with histologically or cytologically confirmed adenocarcinoma or poorly differentiated carcinoma were eligible if no primary tumour was identified after a standardised diagnostic work-up. This consisted of history and physical examination, blood and urine chemistry, chest x-ray, computerised tomography (CT) of the abdomen/pelvis and mammography in women with adenocarcinoma/poorly differentiated carcinoma. Additional radiologic, scintigraphic and endoscopic investigations were directed by relevant symptoms or signs. Hematoxylin-eosin light microscopy was necessarily supplemented by detailed immunohistochemical studies in order to rule out presence of lymphomas, sarcomas, melanomas, prostate cancer and germ cell tumours.
Patient enrollment was stratified for favourable (females with non-mucinous peritoneal carcinomatosis, patients with predominantly nodal disease) and unfavourable (multiple visceral or bony deposits) risk disease. Women with peritoneal carcinomatosis underwent surgical debulking at diagnosis. Patients were eligible if they were chemonaive, their performance status (PS) was 0-2 by Eastern Cooperative Oncology Group (ECOG) criteria, had adequate bone marrow, renal and hepatic function (granulocyte count > 1 500/µl, platelet count > 100 000/µl, serum creatinine ≤ 1.5 mg/dl, bilirubin ≤ 1.5 mg/dl and AST/ALT ≤ 2x-5x the upper limit of normal in the absence or presence of liver metastases respectively) and harboured measurable or evaluable disease. They were excluded from the study in the presence of central nervous system metastases, active coronary artery disease, heart failure, other major medical illnesses, life expectancy shorter than 12 weeks. Patients with isolated axillary adenopathy, isolated squamous cell cervical or inguinal adenopathy and patients with elevated germ cell serum markers (Alpha-fetoprotein, Human Chorionic Gonadotropin) or abnormal serum PSA were excluded from the study. All patients provided written informed consent and were registered with the HeCOG data management central office. The clinical protocol and collateral translational studies were approved by the HeCOG protocol review committee, the scientific board of each hospital and the Bioethics Committee of the Aristotle University of Thessaloniki.
Treatment
Docetaxel at a dose of 75 mg/m2 was administered intravenously by a 30-minute infusion after standard intravenous premedication with ondansetron 8 mg, dexamethasone 16 mg, ranitidine 100 mg and dimethindene 0.1 mg/kg of body weight. On the day before chemotherapy administration patients received oral dexamethasone 8 mg bd. Following docetaxel administration, carboplatin was infused intravenously over 30 minutes at an AUC of 5 mg/ml/min (area under the free carboplatin plasma concentration versus time curve). The Calvert formula was used for carboplatin dosing and creatinine clearance was calculated by means of the Cockcroft-Gault equation Citation[8], Citation[9]. Granulocyte colony-stimulating factor (GCSF) administration was not required per cycle but was allowed for management of febrile neutropenia, protracted grade 4 neutropenia, secondary prophylaxis after previous episodes of febrile neutropenia and for avoidance of treatment delays, according to physician's decision, and was recorded. Erythropoietin administration was allowed for patients with anemia (Hb < 11 gr/dl). Chemotherapy cycles were repeated every three weeks, responding patients being allowed to receive a total of eight courses.
Toxicity and response evaluation
Treatment delays and dose modifications were based on complete blood cell counts taken on Day 1 of each cycle as well as on any organ-specific toxicity occurring at any time during therapy. Retreatment was given upon resolution of any toxicity to grade 1 or less and upon adequate bone marrow function (absolute neutrophil count ANC ≥ 1 500/µl, platelet count ≥ 100 000/µl). In case of grade 3 or 4 thrombocytopenia or non-hematologic toxicity, drug doses were decreased to 75% for docetaxel and to AUC 4 for carboplatin. Toxicity was graded according to the World Health Organisation (WHO) criteria.
Patients were evaluated for efficacy every two cycles with physical examination, appropriate imaging studies and measurement of clinical/superficial lesions. The WHO criteria were used for definition of complete response, partial response, stable and progressive disease Citation[10]. Confirmation of objective responses (complete and partial responses) was performed at least four weeks after their documentation.
Endpoints and statistical issues
The primary endpoint for this study was the objective response rate, while secondary endpoints were toxicity, time to disease progression (TTP), overall survival (OS) and predictive/prognostic significance of clinicopathologic parameters for activity of therapy and patient outcome. Sample size estimation was based on response rate, with accepted type I error of 5%, type II error of 20% and an estimated response rate of 50%. In order to evaluate the effect of age, performance status (PS), baseline abnormal tumour markers, number of metastatic sites, presence of liver metastasis and administered dose intensity of cytotoxic drugs, a univariate logistic regression analysis was performed. Patients’ overall survival and time to disease progression were calculated from the date of chemotherapy initiation to patient death or last contact and patient relapse, death or last contact respectively according to the Kaplan-Meier product limit method Citation[11]. For both survival and TTP, the univariate Cox regression analysis was performed.
Results
Patient characteristics
Forty-seven patients (21 males, 26 females) of a median age of 63 years, mostly asymptomatic or mildly symptomatic with favourable-risk (24) or unfavourable-risk (23) CUP of glandular/undifferentiated carcinoma histology participated in the trial (). Favourable-risk patients had either predominantly nodal disease with limited pulmonary/pleural involvement but absence of disseminated visceral metastases (n = 16) or non-mucinous peritoneal carcinomatosis (all females, n = 8). Only five patients had limited lung or pleural involvement (less than 3 pulmonary nodules of maximal diameter of 3 cm or small-volume asymptomatic pleural effusions) with the bulk of tumour being midline nodal metastases (neck, mediastinum, retroperitoneum). Unfavourable-risk patients harboured systemic visceral metastases (n = 17) with or without mucinous peritoneal carcinomatosis (males n = 6), skin (n = 1) or intestinal wall deposits (n = 2). The majority of patients had metastatic dissemination in less than three organ sites (87%). Patients in the unfavourable risk group tended to experience tumour-associated symptoms more often and harboured bulkier malignant disease.
Table I. Patient and disease characteristics.
By definition, serum concentrations of prostate-specific antigen (PSA), alpha-fetoprotein (AFP) and human chorionic gonadotrophin (HCG) were normal in all accrued patients. Three quarters of all patients (72%) had abnormal serum levels of at least one tumour marker (CA 15-3, CA 19-9, CEA, CA125), half of them exhibiting high concentrations of multiple markers. The most commonly elevated serum marker was CA125 (abnormal in 38% of patients), followed by CA 15-3 (28%), CEA (23%) and CA 19-9 (15%).
Treatment delivery
A total of 221 cycles of chemotherapy were administered in 45 patients (median of 6 cycles per patient). The median number of administered cycles was six in the favourable risk group and four in the unfavourable risk group (). Two patients in the unfavourable group never started chemotherapy because of rapid clinical deterioration due to disease progression but were included in all activity and outcome analyses on an intent-to-treat principle (ITT). Half of the patients completed planned treatment (67% in favourable group, 39% in unfavourable group), the most common cause of treatment discontinuation being disease progression or death from cancer. The administered dose of carboplatin was 93% of the planned dose, while actual dose-intensity of docetaxel was 97% of the planned one. Support with GCSF was applied in a third of the total number of cycles in 13 patients, mainly for maintenance of dose-intensity. Erythropoietin was administered in 20% of cycles in a total of 12 patients for management of anemia.
Table II. Treatment and efficacy data.
Efficacy
From all 47 accrued patients objective tumour regression was observed in 15 (ITT objective response rate of 32%). Of note, one patient in the favorable prognosis group discontinued treatment prior to evaluation, while in the unfavourable-risk group, two patients never received the protocol treatment, two discontinued treatment prior to evaluation and two underwent surgery before chemotherapy and entered the study without measurable/evaluable disease. Accordingly, with tumour regression seen in 15 of 40 evaluable patients, the non-ITT response rate was 37.5%.
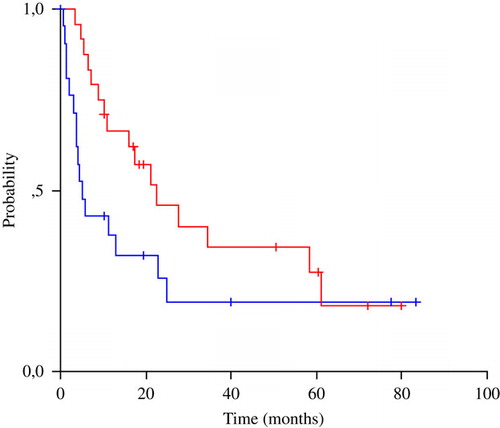
Response rates were 46% in favourable risk and 17% in unfavourable risk patients. Complete remissions occurred in five patients (11%), solely in the favourable-risk group. In particular, three complete remissions occurred in women with peritoneal carcinomatosis and two in patients with predominantly nodal disease. Among the six partial responders in the favourable group, three had peritoneal disease and three nodal deposits. Four patients with multiple visceral metastases obtained a partial remission. Overall, disease control with symptom palliation was achieved in 23 patients (49%), sixteen (67%) among favourable risk and seven (30%) among unfavourable risk subjects. At a median follow-up of 60 months, the median time to progression was 5.5 months and the median overall survival 16 months. TTP and OS were respectively 11.5 and 22.6 months in favourable-risk patients and a poor 3.1 and 5.3 months in unfavourable-risk patients (). Long-term survivors (>4 years) were seen mainly in the favourable group (four patients, two with nodal deposits and two with peritoneal carcinomatosis), though two unfavourable-risk patients also enjoyed long-term disease control. Data on treatment activity and patient outcome are shown in .
Toxicity
Overall the studied regimen was very well tolerated by the majority of 45 treated patients, even those who were less fit (PS 2). Most side-effects were mild to moderate (grades 1-2), easily manageable with appropriate measures and consisted of neutropenia, anemia, fatigue, thrombocytopenia and less commonly diarrhoea, myalgias and serum transaminase elevation. Total alopecia occurred in all patients treated with more than two chemotherapy cycles. With the liberal use of cytokine growth factors, grade 3 or 4 myelosuppression manifested as uncomplicated neutropenia in 26% of patients, anemia in 6% and thrombocytopenia in 4%. Three episodes of febrile neutropenia were documented (7%), all resolving uneventfully with intravenous administration of broad-spectrum antibiotics. Severe diarrhoea, fatigure, myalgias and hepatotoxicity were seen very rarely and were reversible upon institution of treatment breaks and appropriate measures. Only one unfavourable risk group patient opted to quit therapy as a result of clinical deterioration from disease progression and extreme fatigue. No distinct toxicity patterns were apparent in favourable and unfavourable risk patients, except for a two times higher incidence of severe uncomplicated neutropenia in the favourable group. Encountered severe side-effects are summarised in .
Table III. Grade 3 or 4 toxicity data.
Predictive/prognostic factors
Univariate analysis was carried out in order to screen the parameters of patient age, performance status, presence of at least one abnormal serum tumour marker, number of metastatic organ sites, presence of liver metastases, relative dose-intensity of chemotherapy and CUP risk group for predictive or prognostic significance for tumour regression and patient survival (). No parameter was found to significantly predict for response to chemotherapy (p > 0.05). Disease progression was significantly more likely in the presence of mild to moderate symptoms (PS 1or 2, Hazard ratio 2.4, p = 0.01) and only in unfavourable-risk patients, in the presence of abnormal serum markers (Hazard ratio 3.6, p = 0.04) and of multiple metastatic organ sites (Hazard ratio 3.5, p = 0.03). Patients at increased risk of death were those who had a PS of 1 or 2 as opposed to PS 0 (Hazard ratio 3.8, p < 0.001) and those who harboured metastatic deposits in more than one organ sites (Hazard ratio 2.1, p = 0.03). The presence of abnormal serum tumour markers was associated with increased risk of death only in unfavourable-risk patients, while administration of docetaxel at 90% or higher relative dose intensity significantly correlated with prolonged survival in favourable-risk patients (Hazard ratio 0.14, p = 0.01).
Table IV. Prognostic factors for patient outcome.
Discussion
Taxane/platinum-based chemotherapy regimens have been widely used in the management of patients with solid tumours, as they represent a synergistic combination of a mitotic spindle poison with a DNA-damaging agent that proved to be effective and well tolerated. In view of the activity of the regimen in breast, ovarian, head/neck, lung, endometrial, gastric cancer, it was a logical step forward to study its value in treating patients with metastatic tumours as heterogeneous as CUP. Up to date, twelve phase II studies have evaluated taxane-platinum combinations in cancer of unknown primary patients, in a few occasions with the addition of a third cytotoxic drug Citation[12–27]. Response rates ranged from 22 to 48% and median overall survival from 8 to 13 months. Most regimens were relatively well tolerated, though some were associated with a significant risk of myelosuppression, infections and gastrointestinal or neurologic toxicity, especially when a third drug was added. Rates of severe myelosuppresion reported were 12–70%, those of non-hematologic severe side-effects 3–30%, and toxic deaths occurred in 0–5% of patients. Still, both paclitaxel and cisplatin are cumbersome to administer in the outpatient setting as they require prolonged infusion times and intravenous hydration and are associated with additive neurologic toxicity.
A docetaxel/carboplatin regimen offers the convenience of shortened infusion times without need for rigorous hydration schedules and promises lack of additive neurotoxicity. The combination was tested in CUP only once, in a phase II study by Greco et al who reported objective response rates of 22% and a median survival of 8 months Citation[16], Citation[25]. Their study population was of identical size (n = 47) and consisted of fit patients with adenocarcinoma or poorly differentiated carcinoma excluding women with peritoneal carcinomatosis, while the docetaxel dose was lower (65 mg/m2) and that of carboplatin higher (AUC 6). No complete responses were observed, though occasional patients survived longer than two years from treatment start. Half of all patients experienced grade 3 or 4 leukopenia, six as neutropenic fever. Two patients died from sepsis and three discontinued treatment due to toxicity.
Our treatment regimen is extremely convenient as outpatient palliative therapy of patients with incurable, metastatic malignancies, as each cycle was successfully completed within one hour without occurrence of allergic reactions. GCSF was given mainly for secondary prophylaxis after prior febrile neutropenia and for avoidance of treatment delays or dose reductions due to myelosuppression in a third of patients. This strategy probably allowed us to reduce the incidence of severe myelosuppression and neutropenic fever by half in comparison to Greco et al. Non-hematologic toxicity was severe in less than 8% of patients, with an impressive absence of severe or persistent neurologic injury. This finding is in keeping with data from the SCOTROC1 ovarian cancer trial, which showed that docetaxel was associated with neurotoxicity less often than paclitaxel Citation[7]. No toxic deaths occurred and therapy was delivered at an almost ideal relative dose-intensity and number of cycles. The ITT rate of tumour shrinkage of 32% (46% in favourable group versus 17% in unfavourable group), though inferior to the rather optimistic 50% target response rate of the study, is in keeping with published experience and comparable to response rates reported with paclitaxel/platinum combinations, with half of patients enjoying disease control. Remarkably, the rate of complete remission was among the highest reported in the literature (11%), higher than the ones obtained with paclitaxel/cisplatin regimens. However, it was driven exclusively by the favourable-risk group as patients with visceral deposits only rarely enjoyed partial tumour shrinkage of short duration. The inclusion in our study of women with non-mucinous peritoneal carcinomatosis, a good prognosis patient subgroup with chemosensitive disease, constitutes a favourable selection bias that should be acknowledged. The promising regimen activity and encouraging patient survival in the favourable-risk group may be at least partly attributable to the inclusion of these women. Accordingly, any enthusiasm generated by the high complete response rate seen in favourable-risk patients should be moderated by the fact that complete responses are frequently seen in women with non-mucinous peritoneal carcinomatosis with modern chemotherapy regimens Citation[3] and by the selection bias evident in this trial: enrolled patients were young, fit with good organ function reserves and harboured relatively low-volume disease.
Unfortunately, tumour regression or even eradication did not seem to translate to long-term survival, especially in unfavourable-risk patients with visceral metastases. Median time to progression was only 5.5 months for all patients. Median survival, though a satisfactory 23 months in patients with nodal disease or peritoneal carcinomatosis, was a poor 5 months in those with visceral spread. In an effort to validate known prognostic variables and identify new ones, we found good performance status and low volume disease predictive for favourable patient outcome, while docetaxel relative dose-intensity (RDI) was associated with an 86% reduction of the risk of death only in favourable-group patients. These parameters are of known prognostic significance in several patient series, reflecting host and tumour inherent biologic characteristics as well as ability to tolerate toxic treatment and easier cytoreduction of less expanded malignant clones Citation[28]. The significance of docetaxel RDI in a chemosensitive CUP subset probably makes biological sense and is reminiscent of the importance of chemotherapy dose-intensity in lymphomas and germ cell tumours Citation[29], Citation[30]. This observation supports the investigation of dose-escalation or dose-dense approaches in the management of favourable CUP patients.
In conclusion, docetaxel/carboplatin is convenient and safe for outpatient palliative management of patients with CUP. The combination seems to be at least as effective as the more toxic and difficult to infuse paclitaxel/cisplatin regime, though meaningful survival prolongation is seen only in favourable-risk patients. Still, more research is needed in order to consolidate responses, reverse tumour resistance and prevent progression in the majority of patients with visceral metastatic spread. Evidence on the molecular biology profile of CUP is slowly accumulating Citation[31]. As it looks unlikely that empirical cytotoxic drug cocktails will breach the one-year median survival barrier, insights in the biology of CUP are imperatively needed. Such knowledge will allow us to target biomolecules of pivotal importance for the pathophysiology of CUP and alter mechanisms of resistance/survival. Recently, Hainsworth et al. published the first targeted therapy trial results in CUP patients, employing manipulations of the vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) pathways Citation[32]. Though activity was modest, such strategies show the future direction towards which translational research efforts should focus for the control of a highly resistant as well as heterogeneous malignant disease.
Acknowledgements
We would like to thank Mrs Georgia Vourli, Olga Siarabi, Maria Mosxoni for their valuable contribution in data management, statistical and administrative issues.
References
- Pavlidis N, Fizazi K. Cancer of unknown primary (CUP). Crit Rev Oncol Hematol 2005; 54: 243–50
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003; 39: 1990–2005
- Pentheroudakis G, Briasoulis E, Karavasilis V, Fountzilas G, Xeros N, Samelis G, et al. Chemotherapy for patients with two favourable subsets of unknown primary carcinoma: Active, but how effective?. Acta Oncol 2005; 44: 155–60
- Briasoulis E, Tsokos M, Fountzilas G, et al. Bcl2 and p53 protein expression in metastatic carcinoma of unknown primary origin: Biological and clinical implications: A Hellenic Cooperative Oncology Group study. Anticancer Res 1998; 18: 1907–14
- Konecny G. Drug interactions and cytotoxic effects of paclitaxel in combination with carboplatin, epirubicin, gemcitabine or vinorelbine in breast cancer cell lines and tumour samples. Breast Cancer Res Treat 2001; 67: 223–33
- Pentheroudakis G, Razis E, Athanassiadis A, Pavlidis N, Fountzilas G. Paclitaxel-Carboplatin combination chemotherapy in advanced breast cancer: accumulating evidence for synergy, efficacy and safety. Med Oncol 2006; 23: 147–60
- Vasey PA. Role of docetaxel in the treatment of newly diagnosed advanced ovarian cancer. J Clin Oncol 2003; 21(Suppl 10)136s–144s
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
- Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Pavlidis N. Forty years experience of treating cancer of unknown primary. Acta Oncol 2007; 46: 592–601
- Hainsworth J, Erland JB, Kalman LA, Schreeder MT, Greco FA. Carcinoma of unknown primary site: Treatment with 1-hour paclitaxel, carboplatin and extended schedule etoposide. J Clin Oncol 1997; 15: 2385–93
- Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, et al. Carboplatin plus paclitaxel in unknown primary carcinoma: A phase II study. The Hellenic Cooperative Oncology Group study. J Clin Oncol 2000; 18: 3101–17
- Greco FA, Burris HA 3rd, Erland EB, Gray JR, Kalman LA, Schreeder MT, et al. Carcinoma of unknown primary site. Cancer 2000; 89: 2655–60
- Greco FA, Gray J, Burris HA, Erland JB, Morrissey LH, Hainsworth JD. Taxane-based chemotherapy for patients with carcinoma of unknown primary site. Cancer J 2001; 7: 203–12
- Bouleuc C, Saghatchian M, Di Tullio L, Louvet Ch, Levy E, Di Palma M, et al. A multicentre phase II study of docetaxel and cisplatin in the treatment of cancer of unknown primary site. Proc Am Soc Clin Oncol 2001;137b:2298.
- Gothelf A, Daugaard G, Nelausen K. Paclitaxel, cisplatin and gemcitabine in the treatment of unknown primary tumours, a phase II study. Proc ESMO 2002; 25: 88
- Greco FA, Burris HA 3rd, Litchy S, Barton JH, Bradof JE, Richard SP, et al. Gemcitabine, carboplatin and paclitaxel for patients with carcinoma of unknown primary site: A Minnie Pearl Cancer Research Network study. J Clin Oncol 2002; 20: 1651–6
- Mukai H, Watanabe T, Ando M, Katsumata N. Unknown primary carcinoma: A feasibility assessment of combination chemotherapy with cisplatin and docetaxel. Int J Clin Oncol 2003; 8: 23–5
- Greco FA, Rodriguez GI, Shaffer DW, Hermann R, Litchy S, Yardley DA, et al. Carcinoma of unknown primary site: Sequential treatment with paclitaxel/carboplatin/etoposide and gemcitabine/irinotecan. A Minnie Pearl Cancer Research Network phase II trial. The Oncologist 2004; 9: 644–52
- Park YH, Ryoo BY, Choi SJ, Yang SH, Kim HT. A phase II study of paclitaxel plus cisplatin chemotherapy in an unfavourable group of patients with cancer of unknown primary site. Jpn J Clin Oncol 2004; 34: 681–5
- El-Rayes BF, Shields AF, Zalupski M, Heilbrum K, Jain V, Terry D, et al. A phase II study of carboplatin and paclitaxel in adenocarcinoma of unknown primary. Am J Clin Oncol 2005; 28: 152–6
- Hainsworth J, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin and etoposide in advanced poorly differentiated neuroendocrine carcinoma: A Minnie Pearl Cancer Research Network study. J Clin Oncol 2006; 24: 3548–54
- Greco FA, Erland JB, Morrissey LH, Burris HA 3rd, Hermann RC, Steis R, et al. Carcinoma of unknown primary site: Phase II trials with docetaxel plus cisplatin or carboplatin. Ann Oncol 2000; 11: 211–5
- Berry W, Elkordy M, Rourke O M, Khan M, Asmar L. Results of a phase II study of weekly paclitaxel plus carboplatin in advanced carcinoma of unknown primary origin: A reasonable regimen for the community-based clinic?. Cancer Invest 2007; 25: 27–31
- Pouessel D, Culine S, Brecht C, Ychou M, Romieu G, Fabbro M, et al. Gemcitabine and docetaxel as front-line chemotherapy in patients with carcinoma of an unknown primary site. Cancer 2004; 100: 1257–61
- Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of unknown primary site. J Clin Oncol 2002; 20: 4679–83
- Pentheroudakis G, De Bono JS, Kaye SB, Simpson A, Paul J, Brown I, et al. Improved prognosis of patients with intermediate- and poor-risk non seminomatous germ cell tumours by optimising combined treatment. BJU Int 2003; 92: 36–42
- Collette L, Sylvester RJ, Stenning SP, et al. Impact of the treating institution on survival of patients with poor prognosis metastatic non seminomatous germ cell tumours. J Natl Cancer Inst 1999; 91: 839–46
- Pentheroudakis G, Briasoulis E, Pavlidis N. Cancer of unknown primary site: Missing primary or missing biology?. The Oncologist 2007; 12: 418–25
- Hainsworth J, Spigel DR, Farley C, Thompson DS, Shipley DL, Greco FA. Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: The Minnie Pearl Cancer Research Network. J Clin Oncol 2007; 25: 1747–52