To the Editor,
Second malignant neoplasms are a major concern during the long-term follow-up of paediatric patients after successful multi-modality treatment. The only pathogenic factor known to promote development of thyroid cancer in children is radiation, which induces activating rearrangements in the RET gene Citation[1–3].
An eighteen month old boy presented with a large intrathoracic paraspinal mass invading the lower thoracic vertebrae causing spinal cord compression. He had no congenital abnormalities, developmental delays, history of antenatal exposure to radiation or other carcinogens, nor any family history of benign thyroid conditions or malignancy. Haematological examination, bone marrow studies, Serum Alfa-fetoprotein, Human Chorionic Gonadotrophin, urinary Vanillyl-Mandelic Acid, Homo-Vanillic Acid and Dopamine levels, Tc99 MIBG and bone scan were normal. Biopsy from the tumor showed an undifferentiated malignancy composed of small round blue cells with scanty cytoplasm and extensive stroma with necrosis within fibrosis. FISH showed FKHR disrupting translocation, with no EWS disruption. Final diagnosis was Embryonal Rhabdomyosarcoma.
Chemotherapy (International Society of Paediatric Oncology – malignant mesenchymal tumour (SIOP-MMT) 95 protocol) with a six drug schedule (Ifosfamide (cumulative dose – 18 480 mg), Vincristine (10.92 mg), Carboplatin (840 mg), ActinomycinD (2.52 mg), Epirubicin (840 mg) and Etoposide (1 176 mg)), was given for 9 cycles resulting in a radiologic partial response. Thoracotomy and resection of the paraspinal residual tumor revealed a pathological complete response. The patient did not receive adjuvant radiotherapy and was on regular follow-up with normal growth and development. Three CT scans of the thorax were performed during diagnosis, treatment and follow-up.
Six years after completing treatment, a non tender thyroid swelling was detected on follow-up. Ultrasound scan showed a vascular nodule in the left lobe of thyroid and small nodes in the anterior triangle in the left neck. Thyroid function tests were normal. The patient underwent total thyroidectomy and level VI cervical lymph node dissection. Pathological examination revealed a 45 mm well differentiated papillary carcinoma involving the left lobe of the thyroid, with focal capsular and perithyroid fat invasion and with two of six lymph nodes positive. The AJCC2002/TNM stage was pT3pN1aM0. He received post operative thyroid ablation with 1.5GBq of Iodine-131 and remains disease free 12 months later on suppressive dose of thyroxin.
Methods
Sequence analysis
Eight µm sections of the paraffin-embedded PTC sample were stained with Fast Red after deparaffinizing. Regions of tumour tissue were obtained by laser capture micro-dissection, using the Pixcel II LM system and Capsure HS LCM caps (Arcturus Engineering Inc, California, USA). The captured cells were digested in 25 µl of proteinase K buffer, incubated overnight at 55°C, and then incubated at 95°C for 10 minutes to inactivate the proteinase K. Using 10 µl of the DNA solution as template, sequential polymerase chain reactions were performed to amplify exon 15 of B-RAF and exons 11, 13, and 17 of c-kit. The products were purified by agarose gel electrophoresis, sequenced directly with primers used in the amplification step, by automated dideoxy sequencing using Big-Dye Terminator RR mix (Applied Biosystems, California, USA) and analyzed using the Sequencher 4.2.1 program (Gene Codes Corporation, Michigan, USA).
Dual-colour fluorescence In situ hybridization (FISH)
FISH probes were generated from a pool of two overlapping bacterial artificial chromosome (BAC) clones selected from Ensembl genome (http://www.ensembl.org/Homo_sapiens/index.html). To detect RET gene rearrangement, a split-apart probe consisting of BAC clones RP11-818P01 and RP11-696N03 mapped to the telomeric site of RET, and RP11-686A03 and RP11-290103 mapped to the centromeric site of RET was used. BAC DNA was extracted using the Qiagen Plasmid Mini Kit (Qiagen, Crawley, West Sussex, UK) and amplified using the GenomiPhi whole genome amplification kit (WGA Kit, GE Healthcare). The mapping information of each BAC clone was checked using FISH mapping and end sequence techniques according to Lambros et al. Citation[4].
A 2µm section from the paraffin-embedded PTC sample was hybridised. Twenty µl of the split-apart probe for the RET gene were used (RP11-818P01 and RP11-696N03 were labelled with biotin whereas RP11-686A03 and RP11-290103 were labelled with digoxigenine) and subsequently were developed applying a mixture of 1.5 µl of Avidin-cy3 (Sigma, UK) and 1.5 µl of anti-dig FITC diluted in 200 µl PBS/2% BSA for 40 min in the dark at room temperature. After dehydrating, the slides were mounted in an antifade medium (Vector Laboratories, Peterborough, UK) containing 4,6-diamino-2-phenylindole(DAPI) as counterstain. The probes were visualized using a TCS SP2 confocal microscope (Leica, Milton Keynes).
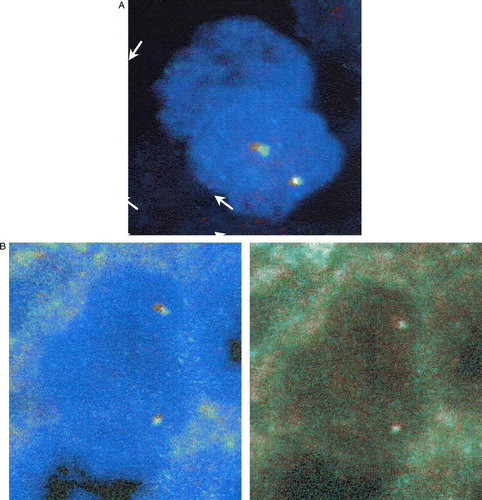
On DNA Sequence Analysis, mutations could not be detected either in B-RAF exon 15, or in the analysed three exons of c-kit, 11, 13, and 17. In an ER/PR-positive tissue sample used as normal control, fluorescence microscopy revealed two sets of red and green signals per nucleus located closely together as expected (A). The PTC sample analyzed showed virtually absent break-apart signals in the RET gene (less than 4% of all analyzed cells), suggesting that the patient's PTC does not harbour any RET gene rearrangements (B).
Figure 1. FISH analysis with RET-specific DNA probes on paraffin sections. A. Status of the proto-oncogene RET in an ER/PR-positive tissue control: A normal cell exhibiting overlapping FISH signals. B. Status of the proto-oncogene RET in the papillary thyroid cancer sample exhibiting overlapping FISH signals (no split-aparts)
Discussion
Induction of secondary thyroid malignancy following exposure to radiotherapy for RMS in childhood is well documented Citation[5], Citation[6], while this complication has not been reported in paediatric patients, following chemotherapy alone. Isolated reports of secondary thyroid malignancy have been published in children after chemotherapy alone for primary malignancies like ALL, Hodgkin's disease, neuroblastoma, osteosarcoma and Ewing's sarcoma Citation[6–9], but not after RMS. This is probably the only reported instance of a childhood PTC as second malignancy after successful treatment for RMS with chemotherapy, as an infant.
The process of thyroid carcinogenesis is believed to be a series of events induced by genetic and environmental factors which alters follicular cell division and growth control Citation[7], Citation[10]. These factors include tumor initiators like radiation and chemicals, which cause DNA damage and promoters like goitrogens, which augment TSH secretion and promote tumor growth. Children who received chemotherapy, especially alkylating agents, DNA intercalators or topoisomerase inhibitors, have a relative risk of 2.7 of developing second primary solid tumors, with a mean latent period of four years Citation[5], Citation[8], Citation[9], Citation[11]. There is no conclusive evidence for increased risk of second thyroid malignancy after previous chemotherapy for childhood tumors Citation[2], Citation[9], Citation[12]. Chemotherapy may result in subclinical hypothyroidism and chronic hypersecretion of TSH, which increases the risk of secondary thyroid oncogenesis Citation[13].
Radiation induced thyroid cancers are almost always papillary and are characterised by the presence of RET/PTC oncogene rearrangement, a subtype limited to thyroid carcinoma in children under 10 years of age Citation[14]. The estimates of the cumulative increased risk of secondary thyroid malignancy after radiotherapy, is 53 to 80 times the general population risk, and is highest among children exposed to radiation before the age of 10 years. A strong linear dose-response curve is reported with a significant risk of second thyroid malignancy even at radiation doses as low as 100 mSv. For a dose of 1 Gy, the estimated odds ratio of childhood thyroid cancer in Chernobyl survivors varied from 5.5 to 8.4 Citation[15], while the excess relative risk of thyroid cancer after paediatric diagnostic radiation exposure has been estimated to be 7.7/Sv Citation[16]. The thyroid organ absorbed dose from CT scan thorax in a child has been calculated to be 550±60×10−5 Gy Citation[17], and a small but finite lifetime cancer mortality risk from diagnostic radiation exposure has been suggested even in infants Citation[18].
RMS and PCT do not share a common genetic predisposition. In our patient, we could not demonstrate rearrangements of RET oncogene, which is the hallmark of radiation related PTC. The protein kinase B-RAF is mutated in about 44% of PTC and is linked to an adverse prognosis Citation[19]. c-kit is a receptor tyrosine kinase important for cell differentiation and growth control of thyroid epithelium and alterations of the c-kit proto-oncogene might play a role in malignant transformation of the thyroid Citation[20]. In our patient, mutations commonly related to PTC like B-RAF or c-kit alterations too could not be detected.
Considering the lack of predisposing factors or a family history of malignancy and the time interval of 5 years between the two malignancies, it is highly likely that thyroid carcinogenesis may have been induced by chemotherapy which caused DNA damage through the alkylating agent ifosfamide, the DNA intercalator Actinomycin D and topoisomerase inhibitors like epirubicin and etoposide, together with a small but significant risk due to radiation exposure from diagnostic imaging.
With higher cure rates and longer survival, paediatric patients are prone to develop second malignancies and warrant a sincere attempt to reduce the dose of chemotherapeutic agents known to induce second malignancy. The danger of developing second thyroid malignancy emphasizes the need for imaging which does not involve exposure to ionizing radiation and lifelong follow-up with regular thyroid examination.
References
- Feinmesser R, Lubin E, Segal K, Noyek A. Carcinoma of the thyroid in children – a review. J Pediatr Endocrinol Metab 1997; 10: 561–8
- Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet 2005; 365: 2014–23
- Black P, Straaten A, Gutjahr P. Secondary thyroid carcinoma after treatment for childhood cancer. Med Pediatr Oncol 1998; 31: 91–5
- Lambros MB, Simpson PT, Jones C, Natrajan R, Westbury C, Steele D, et al. Unlocking pathology archives for molecular genetic studies: A reliable method to generate probes for chromogenic and fluorescent in situ hybridization. Lab Invest 2006; 86: 398–408
- Tucker MA, Jones PH, Boice JD, Jr, Robison LL, Stone BJ, Stovall M, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res 1991; 51: 2885–8
- Vane D, King DR, Boles ET, Jr. Secondary thyroid neoplasms in pediatric cancer patients: Increased risk with improved survival. J Pediatr Surg 1984; 19: 855–60
- Gow KW, Lensing S, Hill DA, Krasin MJ, McCarville MB, Rai SN, et al. Thyroid carcinoma presenting in childhood or after treatment of childhood malignancies: An institutional experience and review of the literature. J Pediatr Surg 2003; 38: 1574–80
- Smith MB, Xue H, Strong L, Takahashi H, Jaffe N, Ried H, et al. Forty-year experience with second malignancies after treatment of childhood cancer: Analysis of outcome following the development of the second malignancy. J Pediatr Surg 1993;28:1342–8; Discussion 1348–9.
- de Vathaire F, Hawkins M, Campbell S, Oberlin O, Raquin MA, Schlienger JY, et al. Second malignant neoplasms after a first cancer in childhood: Temporal pattern of risk according to type of treatment. Br J Cancer 1999; 79: 1884–93
- Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med 1999; 131: 738–44
- de Vathaire F, Francois P, Hill C, Schweisguth O, Rodary C, Sarrazin D, et al. Role of radiotherapy and chemotherapy in the risk of second malignant neoplasms after cancer in childhood. Br J Cancer 1989; 59: 792–6
- van Santen HM, Vulsma T, Dijkgraaf MG, Blumer RM, Heinen R, Jaspers MW, et al. No damaging effect of chemotherapy in addition to radiotherapy on the thyroid axis in young adult survivors of childhood cancer. J Clin Endocrinol Metab 2003; 88: 3657–63
- Paulides M, Dorr HG, Stohr W, Bielack S, Koscielniak E, Klingebiel T, et al. Thyroid function in paediatric and young adult patients after sarcoma therapy: A report from the Late Effects Surveillance System. Clin Endocrinol (Oxf) 2007; 66: 727–31
- Moppett J, Oakhill A, Duncan AW. Second malignancies in children: The usual suspects?. Eur J Radiol 2001; 38: 235–48
- Cardis E, Richardson D, Kesminiene A. Radiation risk estimates in the beginning of the 21st century. Health Phys 2001; 80: 349–61
- Huda W, Atherton JV, Ware DE, Cumming WA. An approach for the estimation of effective radiation dose at CT in pediatric patients. Radiology 1997; 203: 417–22
- Fearon T, Vucich J. Normalized pediatric organ-absorbed doses from CT examinations. AJR Am J Roentgenol 1987; 148: 171–4
- Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001; 176: 289–96
- Salvatore G, De Falco V, Salerno P, Nappi TC, Pepe S, Troncone G, et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res 2006; 12: 1623–9
- Sattler M, Salgia R. Targeting c-Kit mutations: Basic science to novel therapies. Leuk Res 2004; 28(Suppl 1)S11–S20