Abstract
Introduction. We prospectively evaluated concomitant radiotherapy and chemotherapy for advanced bladder cancer in a phase II EORTC trial to test whether it could be further studied as a potential treatment of bladder cancer. Patients and methods. Patients up to 75 years of age with invasive transitional-cell carcinoma of the bladder up to 5 cm, stage pT2 to pT3b, N0M0, without residual macroscopical tumour after transurethral excision were eligible. Radiotherapy consisted of 2 fractions of 1.2 Gy daily up to 60 Gy delivered in a period of 5 weeks. During the first and the last week, cisplatin 20 mg/m2/day and 5 FU 375 mg/m2/day were given concomitantly. Results. The study was interrupted early due to poor recruitment. Nine patients of the originally 43 planned were treated. Mean age was 63 years. Five patients had tumour stage pT2, 1 stage pT3a and 3 stage pT3b. All patients completed radiotherapy and chemotherapy as scheduled. Only one grade 3 and no grade 4 toxicity was seen. All patients were evaluated 3 months after treatment: eight patients had no detectable tumour and one had para-aortic lymph nodes. During further follow-up, a second patient got lymph node metastases and two patients developed distant metastases (lung in the patient with enlarged lymph nodes at the first evaluation and abdominal in one other). Those three patients died at respectively 19, 14, and 18 months after registration. Late toxicity was limited and often temporary. After 26 to 57 months of follow-up, no local recurrences were seen. Six patients remained alive without disease. Discussion. Despite the small cohort, this combination of concomitant chemotherapy and accelerated hyperfractionated radiotherapy for invasive bladder cancer seemed to be well tolerated and to result in satisfactory local control with limited early and late toxicity. It could therefore be considered for study in further clinical trials.
Muscle invasive bladder cancer is a potentially lethal malignant disease. Depending on tumour grade and stage, the risk of developing metastases is between 20–50%. Radical cystoprostatectomy is the standard treatment with 80% loco regional control and 50–60% long-term disease free survival Citation[1]. However, it may induce considerable urinary and sexual side effects. Elderly patients are also often not suitable for surgery. Therefore, effective alternative treatments are needed.
External radiotherapy alone has been used for many years for patients who are not candidates for radical surgery and gives fairly acceptable local control rates of 31–59% and 5 years disease free survival of 35–40% Citation[2]. A meta-analysis of prospective trials showed only a modest improvement in survival for primary and no advantage for adjuvant chemotherapy Citation[3], Citation[4]. A number of small studies combining chemotherapy with external radiotherapy showed promising results Citation[5–16].
Building on this background, the EORTC Radiation Oncology Group started a prospective phase II trial to evaluate the toxicity and the tumour response of a combination treatment consisting of accelerated hyperfractionated radiotherapy with concomitant chemotherapy after a thorough transurethral resection, as a first step towards designing a prospective randomized phase III trial Citation[17]. The study has been carried out with approval of the ethics committee of the participating institutes and in agreement with the laws and regulations.
Patients and methods
The major eligibility criteria were age less or equal to 75 years; biopsy proven muscle invasive stage pT2 to pT3b, N0M0 transitional cell carcinoma of the bladder with a maximum dimension of 5 cm; microscopically complete transurethral resection; WHO performance status ≤2; absence of contra-indications for the combined modality treatment including a satisfactory renal function (GFR > 60 ml/mn) and the absence of hydronephrosis and informed consent. The primary endpoints were the occurrence of grade III en IV acute toxicity during and shortly after the treatment and the tumour response as evaluated by cystoscopy and biopsies 3 months after completion of treatment. Secondary endpoints were the feasibility of the treatment; disease free survival; late toxicity and overall survival. It was planned to enter 43 patients to exclude a recurrence-free rate of 60% or less and an acute toxicity rate greater or equal to 5% with 90% power at the 1-sided 10% false positive error rate.
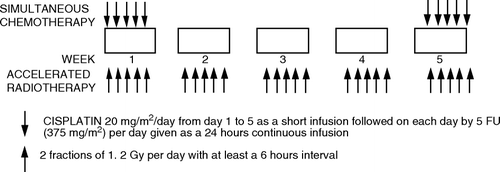
Sixty Gy of radiotherapy were delivered over 5 weeks, 2 fractions daily at a minimum interval of 6 hours, with 40.8 Gy in 34 fractions to the entire bladder and the internal iliac lymph nodes followed by a boost of 19.2 Gy in 16 fractions to the entire bladder with a margin of 2 cm. Treatment prescription and delivery had to comply with the ICRU guideline 50 Citation[18]. A box technique using 3 or 4 fields with appropriate wedges and shielding was to be used and full 3-D treatment planning based on a CT scan and portal imaging were mandatory. The bladder should be emptied prior to each radiotherapy fraction. During the first and the fifth week of treatment a continuous infusion with 5 fluoro-uracil during 5 days was given on an out-patient basis with the use of a pump connected to an implanted device. Daily cisplatin was given as a short infusion (). After treatment, patients were evaluated 3 monthly for the first two years and 6 monthly thereafter. The evaluation consisted of a cystoscopy with biopsies at every visit and a chest x-ray and abdominal CT-scan or MRI every 6 months for the first year and yearly thereafter.
Figure 1. Schematic representation of the treatment schedule.Simultaneous chemotherapy: cisplatin 20 mg/m2/day from day 1 to 5 as a short infusion followed on each day by 5 FU (375 mg/m2) per day given as a 24 hours continuous infusion. Accelerated radiotherapy: 2 fractions of 1.2 Gy per day with at least a 6 hours interval.
Results
Between January 1999 and April 2001, nine patients were registered by two institutes (Tilburg and Bordeaux). In view of this slow accrual, the EORTC decided to close the trial prematurely. We report here on the nine patients treated in the protocol, with a follow-up of 27 to 56 months.
All patients were eligible. Five had tumour stage T2, one stage T3a and three stage T3b. All patients completed the combined modality treatment according to the schedule. Only one case of grade III toxicity was seen (urinary infection complicated by sepsis) and no grade IV toxicity was reported. At the 3-month post-treatment assessment, eight of nine patients were free of disease and one had developed a lymph node metastasis in the para-aortic region (outside of the radiotherapy portals). During follow-up, this patient developed further metastatic disease in the lungs, another patient developed lymph node metastases outside of the radiotherapy fields and one patient developed distant metastasis in the abdomen. These three patients all died of bladder cancer after respectively 14, 19 and 20 months. At the moment of evaluation, six patients were disease free of whom four without late toxicity.
Late toxicity consisted of cystitis in three patients, of which two were temporarily; haematuria in two patients of which one recovered; two cases of temporary pollakisuria; peripheral neuropathy in two patients, one of which recovered and fatigue and urethral stenosis in one patient each.
Discussion
An overview of the publications on combined modality treatment for muscle invasive transitional cell carcinoma of the bladder is given in . Only two phase III trial are reported. In the RTOG trial 123 patients were included to evaluate the value of primary chemotherapy Citation[19]. Coppin et al. reported on a prospective randomized trial with 99 patients to evaluate the addition of concurrent cisplatin (100 mg/m2 at 2-week intervals for three cycles) to preoperative or definitive radiation therapy. After a median follow-up of 6.5 years, the pelvic relapse rate in the two groups was significantly reduced by concurrent cisplatin without any influence on overall survival Citation[20]. Accelerated radiotherapy is shown to be feasible but probably not more effective than conventionally fractionated radiotherapy Citation[2], Citation[21–23].
Table I. Overview of series of combined modality treatment for muscle invasive bladder cancer.
The results for the nine patients recruited in this phase II EORTC trial appear comparable to the results of the other trials and deserve some attention because of the quality of the trial and its data. All six patients who were still alive at the last follow-up are free of disease and have a functional bladder.
Initially, 17 departments from 5 countries agreed to participate in this trial with an estimated yearly accrual of more than 85 patients. We were able to identify by querying the centres that the slow accrual most often resulted from lack of communication between the urology and radiation oncology departments. Furthermore, some of the original inclusion criteria were very restrictive (requiring unifocal tumour ≤5 cm, classified pT2 to pT3b with no residual masses at the end of TURB) and few patients would fulfil them all. An amendment to allow multifocal disease was implemented, but very late and failed to boost the accrual and to prevent the premature closure of the trial.
Recently, a consensus meeting of experts in the treatment of bladder cancer was organized by the Société Internationale d'Urologie to develop an international consensus about the optimal use of radiotherapy, alone or in combination with surgery and chemotherapy, in the radical treatment of patients with bladder cancer. They concluded that radiotherapy is an effective treatment for selected patients with bladder cancer with long-term disease control and preservation of normal bladder function. All newly diagnosed patients should be assessed in a multidisciplinary setting, where the relative merits of surgery, radiotherapy, and chemotherapy can be considered on an individual basis with the aim of optimizing overall outcomes Citation[24].
This EORTC phase II trial failed to meet its recruitment target. Although it is difficult to draw any firm conclusions based on only nine patients, the results suggest that combined modality treatment with accelerated hyperfractionated radiotherapy and concomitant chemotherapy for muscle invasive transitional cell carcinoma seemed well tolerated and resulted in a satisfactory local control in these nine patients. Therefore, in view of other published data, we suggest- that this approach might be considered a reasonable starting point for further investigations of bladder sparing approaches as an alternative to a radical cystectomy.
Acknowledgements
This publication was supported by grants number 5U10 CA11488-27 through 5U10 CA11488-37 from the US National Cancer Institute (Bethesda, Maryland, USA). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
This research project was supported by a grant from the Koningin Wilhelmina Fonds/Kankerbestrijding (Dutch Cancer League, The Netherlands).
References
- Scher HI. New approaches to the treatment of bladder cancer. N Engl J Med 1993; 329: 1420–1
- Widmark A, Flodgren P, Damber JE, Hellsten S, Cavallin-Stahl E. A systematic overview of radiation therapy effects in urinary bladder cancer. Acta Oncol 2003; 42: 567–81
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:189–201.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
- Arias F, Dominguez MA, Martinez E, Illarramendi JJ, Miquelez S, Pascual I, et al. Chemoradiotherapy for muscle invading bladder carcinoma. Final report of a single institutional organ-sparing program. Int J Radiat Oncol Biol Phys 2000; 47: 373–8
- Arias F, Duenas M, Martinez E, Dominguez MA, Illarramendi JJ, Villafranca E, et al. Radical chemoradiotherapy for elderly patients with bladder carcinoma invading muscle. Cancer 1997; 80: 115–20
- Chauvet B, Davin JL, Alfonsi M, Berger C, Vincent P, Reboul F. Conservative treatment of bladder cancers with radiotherapy and concurrent chemotherapy: Results and perspectives. Cancer Radiother 1998; 2: 499–504
- George L, Bladou F, Bardou VJ, Gravis G, Tallet A, Alzieu C, et al. Clinical outcome in patients with locally advanced bladder carcinoma treated with conservative multimodality therapy. Urology 2004; 64: 488–93
- Hagan MP, Winter KA, Kaufman DS, Wajsman Z, Zietman AL, Heney NM, et al. RTOG 97-06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 665–72
- Housset M, Maulard C, Chretien Y, Dufour B, Delanian S, Huart J, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: A prospective study. J Clin Oncol 1993; 11: 2150–7
- Hussain SA, Stocken DD, Peake DR, Glaholm JG, Zarkar A, Wallace DM, et al. Long-term results of a phase II study of synchronous chemoradiotherapy in advanced muscle invasive bladder cancer. Br J Cancer 2004; 90: 2106–11
- Peyromaure M, Slama J, Beuzeboc P, Ponvert D, Debre B, Zerbib M. Concurrent chemoradiotherapy for clinical stage T2 bladder cancer: Report of a single institution. Urology 2004; 63: 73–7
- Sangar VK, McBain CA, Lyons J, Ramani VA, Logue JP, Wylie JP, et al. Phase I study of conformal radiotherapy with concurrent gemcitabine in locally advanced bladder cancer. Int J Radiat Oncol Biol Phys 2005; 61: 420–5
- Shipley WU, Zietman AL, Kaufman DS, Coen JJ, Sandler HM. Selective bladder preservation by trimodality therapy for patients with muscularis propria-invasive bladder cancer and who are cystectomy candidates–the Massachusetts General Hospital and Radiation Therapy Oncology Group experiences. Semin Radiat Oncol 2005; 15: 36–41
- Sumiyoshi Y. Chemoradiotherapy as a bladder-preservation approach for muscle-invasive bladder cancer: Current status and perspectives. Int J Clin Oncol 2004; 9: 484–90
- Venturini M, Merlano M, Michelotti A, Martorana G, Curotto A, Scarpati D, et al. Neoadjuvant or definitive alternating chemotherapy and radiotherapy for infiltrating bladder cancer. Am J Clin Oncol 1989; 12: 63–7
- Bolla M. A phase II feasibility study of combined accelerated external radiation and chemotherapy with 5FU and cisplatin following transurethral resection in patients with muscle invasive transitional carcinoma of the bladder T2-T3, N0, M0 (UICC 1992). EORTC trial 22971. EORTC Data Center, Brussels, 1998.
- International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy. ICRU Report 50. Bethesda, 1993.
- Shipley WU, Winter KA, Kaufman DS, Lee WR, Heney NM, Tester WR, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89–03. J Clin Oncol 1998; 16: 3576–83
- Coppin CM, Gospodarowicz MK, James K, Tannock I, Zee B, Carson J, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1996; 14: 2901–7
- Cowan RA, McBain CA, Ryder WD, Wylie JP, Logue JP, Turner SL, et al. Radiotherapy for muscle-invasive carcinoma of the bladder: Results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. Int J Radiat Oncol Biol Phys 2004; 59: 197–207
- Majewski W, Maciejewski B, Majewski S, Suwinski R, Miszcyk L, Tarnawski R. Clinical radiobiology of stage T2-T3 bladder cancer. Int J Radiat Oncol Biol Phys 2004; 60: 60–70
- Pos FJ, Hart G, Schneider C, Sminia P. Radical radiotherapy for invasive bladder cancer: What dose and fractionation schedule to choose?. Int J Radiat Oncol Biol Phys 2006; 64: 1168–73
- Milosevic M, Gospodarowicz M, Zietman A, Abbas F, Haustermans K, Moonen L, et al. Radiotherapy for bladder cancer. Urology 2007; 69(Suppl): 80–92