Abstract
Background. In prostate cancer, gamma-aminobutyric acid (GABA) has been previously reported to increase cellular proliferation via the ionotropic GABAa receptor (GABAar) and to promote cellular invasiveness via the metabotropic GABAb receptor. Methods. In this study, we have investigated, by immunohistochemistry, GABAar levels in 12 normal human prostate, 13 benign prostatic hyperplasia (BPH) and 148 human prostate cancer specimens. We have also examined the effect of several GABA agonists and antagonists on the in vitro proliferation of four human prostate cancer cell lines: LNCaP, MDA-PCA-2b, DU145 and PC3. Results. GABAar immunoreactivity was present in the stroma of ∼75% of the normal and BPH specimens, and in 95% of the prostate cancer specimens. Also, low to moderate GABAar staining was observed in the acinar epithelium of 50 (33%) prostate cancer specimens. No correlation was observed between GABAar staining and patient age, Gleason Sum or TNM stage. A GABAa agonist isoguvacine, at doses between 5–50 ug/ml (31–310 uM), stimulated the proliferation of all four human prostate cancer cell lines, tested. Baclofen, a GABAb agonist (up to 50 ug/ml, 234 uM) had no effect on growth. Also, at concentrations up to 100 ug/ml, GABA antagonists, bicuculline (223 uM), picrotoxin (166 uM) and saclofen (400 uM), did not have significant growth-inhibitory effects. However, dihydroergotoxine, which binds the GABAar chloride ion-channel, inhibited cellular proliferation (IC50 18–38 uM). Conclusions. These data indicate frequent expression of GABAar in prostate cancer and support a role for GABAar in the proliferation of prostate cancer cells.
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in mammals Citation[1]. GABA can be synthesized from glutamate by the enzyme glutamate decarboxylase (GAD). GABA activity is mediated by GABAa and GABAc ionotropic receptors, which are ligand-gated chloride channels as well as by metabotropic GABAb receptors (GABAbrs) which are coupled to G-protein. GABA can function as a trophic factor during nervous system development, by controlling cell proliferation, migration, differentiation, synapse maturation and cell death Citation[1]. Many peripheral, non-neuronal tissues contain GABA receptors and GABA is known to have a role in the development and maturation of tissues outside the nervous system Citation[2], Citation[3].
GABA activity has been previously associated with prostate cancer metastasis Citation[4]. Patients with prostate cancer metastasis had higher prostate GABA and GAD levels compared to those without metastasis and patients with benign prostatic hyperplasia (BPH) Citation[4]. GABA, acting via the metabotropic GABAbr, elevated the invasive ability of the C4-2 human prostate cancer cell line Citation[4], by increasing the production of matrix metalloproteinases. GABA has also been implicated in the proliferation of prostate cancer cells Citation[5]. Mouse prostate neuroendocrine cancer (PNEC) cells were found to be enriched for GABA and GABAar subunits Citation[5]. GABAar activation can be excitatory in the developing brain as well as in certain mature neurons Citation[6], Citation[7]. In this study, we demonstrate the expression and activity of GABAar in human prostate cancer.
Material and methods
Immunohistochemistry was done as previously described Citation[8]. Twelve normal prostate, 13 BPH and 148 prostate cancer specimens on tissue microarray slides were obtained from the Cooperative Prostate Cancer Tissue Resource and the Cooperative Human Tissue Network under the tissue array program of the National Cancer Institute, The National Institutes of Health (Bethesda, MD, USA). Cancer tissue was from radical prostatectomy specimens. Control non-neoplastic tissue was from BPH cases and control non-diseased, normal tissue was from organ donor prostates. Median patient age was 65 years (range 44–79 years). Gleason sum for the 148 prostate cancer specimens was 5 for 8 (5%), 6 for 48 (32%), 7 for 76 (51%), 8 for 11 (7%) and 9 for 5 (3%). TNM stage for the 148 prostate cancer patients was 2a for 20 (14%), 2b for 78 (53%), 3a for 26 (18%) and 3b for 24 (16%); data for the evaluation of regional lymph nodes was N0 = 128 (86%), N1 = 5 (3%), NX = 15 (10%); data for the evaluation of distant metastasis was M0 = 54 (36%), M1 = 0 (0%), MX = 94 (64%).
Primary antibody was anti-GABAar rabbit polyclonal antibody directed against the GABAar α-6 subunit (3 ug in 300 uL PBS, Chemicon, Temecula, CA). Secondary antibody was horseradish peroxidase–conjugated, goat anti-rabbit antibody (1:150 dilution, BioRad, Hercules, CA). Freshly prepared 3,3′-diaminobenzidine (DAB, Sigma Fast Tablets, St. Louis, MO) was used as the substrate for HRP. Incubation of tissue specimens with secondary antibody alone gave no staining. Immunostaining levels were visually scored as described in . Two primary prostate cancer specimens were provided for each patient on the TMA slide and average immunostaining scores were used. Cell extracts were prepared and Western blotting was done as previously described Citation[9]. 75 ug of cell extract protein was loaded per lane and the primary antibody (anti-GABAar α-6 subunit, rabbit polyclonal antibody, Chemicon) was used at a dilution of 1:1000 (1.5 ug/ml). The same primary antibody was used for immunohistochemistry and Western blotting. In addition to the four human prostate cancer cell lines, extracts of three human colon cancer cell lines (SW116, LoVo and LS174t, from the American Type Culture Collection [ATCC]) were also subjected to Western blotting.
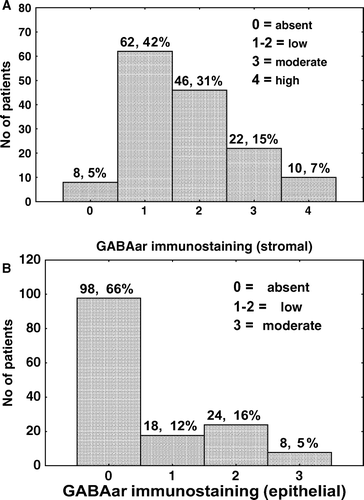
Figure 1. Levels of stromal (top) and epithelial (bottom) GABAar immunostaining in 148 human prostate cancer specimens.
Six human prostate cell lines (from the ATCC) were studied: two immortalized “normal” prostate epithelial cell lines, RWPE-1 and RWPE-2, two androgen-unresponsive prostate cancer cell lines DU145 and PC3, two androgen-responsive prostate cancer cell lines LNCaP and MDA-PCA-2b. Cell lines were maintained in Leibovitz's (L) medium (Gibco, Rockville, MD, USA) containing 100 units/ml penicillin G, 100 ug/ml streptomycin, 0.25 ug/ml amphotericin B and supplemented with 20% Hams F-12, 5 ug/ml insulin, 100 ug/ml transferrin, 30 nM sodium selenite, 5% fetal bovine serum. To study the growth-stimulatory effect of GABA receptor agonists (isoguvacine hydrochloride and baclofen, purchased from Tocris, Ellisville, MO), human prostate cell lines were plated at 7 000 cells per well in 96-well plates in supplemented L-medium. Next day cells were changed to unsupplemented L-medium and allowed to ‘starve’ for 48 h. Cells were then treated with the receptor agonists in unsupplemented L-medium for 72 h and cell numbers determined using calcein AM (Molecular Probes, Eugene, OR).
To study the effect of GABA receptor antagonists (bicuculline methochloride, picrotoxin and saclofen, from Tocris) and a GABA chloride channel modulator (dihydroergotoxine mesylate, from Tocris), on cellular proliferation, human prostate cancer cell lines were plated at 15 000 cells per well in 96-well plates in supplemented L-medium. The following day cells were treated with the receptor antagonists or dihydroergotoxine in unsupplemented L-medium. After 72 h incubation, cell numbers were determined using calcein AM.
Results
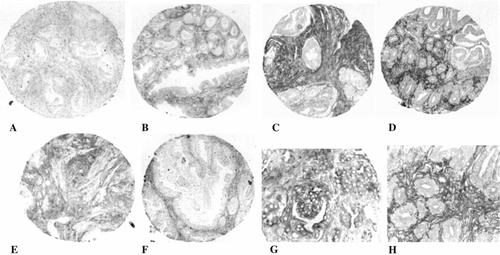
GABAar immunoreactivity was frequently seen in the stromal compartment of prostate cancer specimens (, top and A-D, H). GABAar immunostaining was also found in the stroma of 9 of 12 (75%) normal prostate (E) and in 10 of 13 (77%) BPH specimens (F). GABAar immunoreactivity was infrequently seen in the acinar epithelium of normal prostate (1 of 12, 8%) and BPH (1 of 13, 8%) specimens, but was observed in 50 (33%) prostate cancer specimens (, bottom and G). GABAar staining was observed in some, but not all, prostatic intraepithelial neoplasia (PIN) lesions.
Figure 2. GABAa receptor (GABAar) immunostaining in human prostate specimens. Representative prostate cancer specimens showing low (A), moderate (B) and high (C, D) stromal immunostaining. GABAar immunoreactivity in the stroma of one normal (E) and one BPH (F) specimen. GABAar immunostaining in the acinar epithelium of a prostate cancer specimen (G). Magnification 100×(A-F) and 400×(G, H). A magnified region (400×) within D is shown in H.
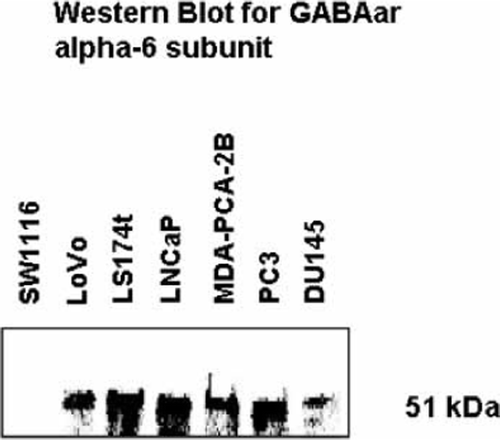
Western blotting of cell extracts from the four prostate cancer cell lines, using the same antibody (directed against GABAar α-6 subunit) as used for immunohistochemistry, detected a ∼51 kDa protein (). Since GABA activity has been previously reported in colon cancer, cell extracts of three human colon carcinoma cell lines were also examined. The GABAar α-6 subunit was present in LoVo and LS174t, but was absent in the SW1116 cell line ().
Figure 3. Western blot of cell extracts from 4 human prostate cancer cell lines (LNCaP, MDA-PCA-2B, PC3 and DU145) and 3 human colon carcinoma cell lines (SW116, LoVo and LS174t), using anti-GABAa receptor (α-6 subunit) antibody.
The GABAa agonist isoguvacine, at doses between 5–50 ug/ml (31–310 uM), stimulated the proliferation of all four human prostate cancer cell lines, with 40–70% maximal growth-stimulation observed, depending on the cell line (, top). Baclofen, a GABAb agonist, at doses up to 50 ug/ml (234 uM) had no significant effect on the growth of any of the four human prostate cancer cell lines examined (not shown). Isoguvacine, at concentrations between 5 and 50 ug/ml, did not have any effect on the growth of two immortalized “normal” prostate epithelial cell lines, RWPE-1 and RWPE-2.
Figure 4. Effect of isoguvacine (top) and dihydroergotoxine (bottom) on the proliferation of four human prostate cancer cell lines.
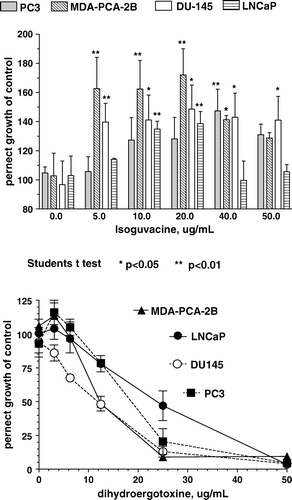
At concentrations up to 100 ug/ml GABAa antagonists, bicuculline (223 uM), and picrotoxin (166 uM) as well as the GABAb antagonist saclofen (400 uM), did not have significant growth-inhibitory effects (not shown). Dihydroergotoxine, which can modulate GABAar chloride ion-channel activity Citation[10], Citation[11], inhibited the proliferation of all four human prostate cancer cell lines (, bottom), with half-maximal growth-inhibition observed between 12 and 25 ug/ml (18–38 uM).
Discussion
Autoradiographic localization of GABAar, using 3H-muscimol, in sections of the normal rat prostate has been previously reported Citation[12]. In the human, we have found GABAar immunostaining in the stroma of normal, BPH and prostate cancer specimens. We have also found GABAar immunoreactivity in the acinar epithelium of human prostate cancer specimens and in four human prostate cancer cell lines. There was no significant correlation observed between GABAar staining (stromal or epithelial) and patient age, Gleason Sum or TNM stage. A GABAa agonist, isoguvacine, stimulated the growth of all four of these cell lines. A significant increase compared to normal tissue, in the expression of GABA and GAD, has been reported in breast, gastric and colorectal cancer Citation[13–16]. Also, overexpression of the GABAar π subunit has been reported in pancreatic carcinoma Citation[17].
Ionotropic GABA receptors consist of a combination of protein subunits from 8 related families: α 1–6, β 1–3, γ 1–3, δ, ε, π, θ and ρ 1–3 Citation[18–20]. GABAars are pentameric, generally containing 2α, 2β and 1γ subunit, with α and β subunits required for activation by GABA. GABAc receptors appear to be homomeric and are known to contain ρ subunits Citation[21]. Generally, GABAars are bicuculline- and picrotoxin-sensitive and GABAc receptors are bicuculline-insensitive, but picrotoxin-sensitive. Bicuculline and picrotoxin failed to inhibit growth of any of the four prostate cancer cell lines, suggesting that these cells have ionotropic GABA receptors with uncommon pharmacological characteristics. The presence of different subunit combinations can confer distinct pharmacological properties to GABAars Citation[22–25]. Further examination of the subunit composition and pharmacological behavior of iontropic GABA receptors in prostate cancer cells is warranted.
Acknowledgements
This work was supported, in part, by SC INBRE (South Carolina IDeA Network of Biomedical Research Excellence), grant number RR16461.
References
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition?. Nat Rev Neurosci 2002; 3: 715–27
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 2002; 213: 1–47
- Gilon P, Remacle C, de Varebeke J, Pauwels G, Hoet JJ. GABA content and localization of high-affinity GABA uptake during the development of the rat pancreas. Cell Mol Biol 1987; 33: 573–85
- Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, et al. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res 2003; 63: 8090–6
- Ippolito JE, Merritt ME, Backhed F, Moulder KL, Mennerick S, Manchester JK, et al. Linkage between cellular communications, energy utilization, and proliferation in metastatic neuroendocrine cancers. Proc Natl Acad Sci USA 2006; 103: 12505–10
- Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci 2002; 3: 728–39
- Stein V, Nicoll RA. GABA generates excitement. Neuron 2003; 37: 375–8
- Abdul M, Hoosein NM. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett 2002; 186: 99–106
- Diaz M, Abdul M, Hoosein NM. Modulation of neuroendocrine differentiation in prostate cancer by interleukin-1 and -2. The Prostate (Suppl.) 1998; 8: 32–6
- Tvrdeic A, Pericic D. Dihydroergotoxine modulation of the GABAA receptor-associated Cl- ionophore in mouse brain. Eur J Pharmacol 1992; 221: 139–43
- Tvrdeic A, Pericic D. Dihydrogenated ergot compounds bind with high affinity to GABAA receptor-associated Cl- ionophore. Eur J Pharmacol 1991; 202: 109–11
- Napoleone P, Bronzett E, Cavallotti C, Amenta F. Predominant epithelial localization of type A gamma-aminobutyric acid receptor sites within rat seminal vesicles and prostate glands. Pharmacology 1990; 41: 49–56
- Opolski A, Mazurkiewicz M, Wietrzyk J, Kleinrok Z, Radzikowski C. The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J Exp Clin Cancer Res 2000; 19: 383–90
- Matuszek M, Jesipowicz M, Kleinrok Z. GABA content and GAD activity in gastriccancer. Med Sci Monit 2001; 7: 377–81
- Kleinrok Z, Matuszek M, Jesipowicz J, Matuszek B, Opolski A, Radzikowski C. GABA content and GAD activity in colon tumors taken from patients with colon cancer or from xenografted human colon cancer cells growing as s.c. tumors in athymic nu/nu mice. J Physiol Pharmacol 1998; 49: 303–10
- Maemura K, Yamauchi H, Hayasaki H, Kanbara K, Tamayama T, Hirata I, et al. Gamma-amino-butyric acid immunoreactivity in intramucosal colonic tumors. J Gastroenterol Hepatol 2003; 18: 1089–94
- Johnson K, Haun RS. The gamma-aminobutyric acid receptor π subunit is overexpressed in pancreatic adenocarcinomas. J Pancreas 2005; 6: 136–42
- Hevers W, Luddens H. The diversity of GABA A receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol 1998; 18: 35–86
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: A brief history. Brit J Pharmacol 2006; 147: S109–S119
- Cascio M. Modulating inhibitory ligand-gated ion channels. AAPS J 2006; 8: E353–E361
- Boue-Garbot E., Taupignon A., Tramu G., Garret M. Molecular and electrophysiological evidence for a GABAc receptor in thyrotropin-secreting cells. Endocrinology 2000; 14: 1627–32
- Burt DR, Kamatchi GL. GABAa receptor subtypes: From pharmacology to molecular biology. FASEB J 1991; 5: 2916–23
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAa receptors: Discrete binding sites underlie subtype specificity. Nat Neurosci 2003; 6: 362–9
- Hedblom E, Kirkness EF. A novel class of GABAa receptor subunit in tissues of the reproductive system. J Biol Chem 1997; 272: 15346–50
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAa receptor alpha6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the alpha6 gene. J Neurosci 2006; 26: 3357–64