Abstract
Background and purpose. Ultrasound has been used successfully to differentiate benign and malignant breast lesions. The aim of this study was to investigate the correlation between ultrasound and prognostic indicators in breast cancer such as histological type, tumor grade, and biological markers. Materials and methods. Ultrasound findings (shape, margin, orientation, boundary, echo pattern, posterior acoustic feature, and presence of calcifications) of 458 breast cancers were analyzed and correlated with the tumor type, tumor grade, and biological markers by univariate and multivariate logistic regression analyses. The biological markers were estrogen receptor, progesterone receptor, and HER-2/neu. Results. Invasive cancers displayed more frequently an irregular shape, a not parallel orientation, and a hypoechoic or complex echo pattern than carcinoma in situ cases (p<0.05). Poorly differentiated invasive cancers had more frequently not circumscribed margins, an abrupt boundary, and a hypoechoic or complex echo pattern than moderately/well differentiated cancers (p<0.05). Estrogen or progesterone receptor negative cancers more often displayed a hypoechoic or complex echo pattern and HER-2/neu positive cancers had more calcifications (p<0.05). Conclusion. Ultrasound pattern is correlated with tumor type, tumor grade, and biological markers in breast cancers and it may be useful for prediction of prognosis.
Histological tumor type, grade, and staging are standard prognostic indicators in breast cancer patients Citation[1]. A breast cancer is drug-sensitive, and many drugs have activity. Citation[2]. Biological markers can be used for prediction of the clinical response to medical treatment and for prediction of prognosis Citation[3]. Estrogen receptor (ER), progesterone receptor (PR), and human epithelial growth factor receptor (HER) are common biological markers. ER and PR are localized in the nuclei of epithelial cells and their presence is predictive for response to hormonal therapy Citation[4]. The HER-2/neu gene has a key role in the HER family and this gene regulates normal cell growth. Trastuzumab (Herceptin®) preferentially targets cells that overexpress HER-2/neu Citation[5].
Breast ultrasound is widely used as a diagnostic modality for evaluating clinical or radiological suspected abnormalities Citation[6], Citation[7] and is an effective screening modality for detecting occult breast cancers in dense breasts Citation[7–9]. Stavros et al. Citation[10] reported that ultrasound has high sensitivity and negative predictive value for diagnosing breast cancers, 98.4% and 99.5%, respectively, and recent advances in ultrasound technology and transducer design permit greater spatial and contrast resolution.
Thus, we investigated correlations between breast ultrasound findings and prognostic indicators in breast cancer such as histological tumor type, tumor grade, and biological markers.
Materials and methods
Patients
This study was approved by the institutional review board for human investigation. The soft copy ultrasound examinations of 458 consecutive patients with primary breast cancer were retrospectively evaluated. All cancers were examined from January 2002 to May 2006 and were histologically proven: 435 invasive cancers (389 ductal carcinoma, 20 lobular carcinoma, and 26 others) and 23 DCIS cases. Patient characteristics are shown in . All patients were female and the ages of the patients ranged from 25 to 87 years (mean age, 56 years). On histological examination, the tumor size ranged from 4 to 125 mm (mean size, 21 mm) and 162 of 458 patients had lymph node metastases.
Table I. Patient characteristics.
Ultrasound
All patients were examined using a Logiq9 unit (General Electronic Medical Systems, Milwaukee, WI USA) or a HDI 5000 unit (Advanced Technology Laboratories, Bothell, WA USA) using a broad-bandwidth (14–5 MHz) and a linear scanhead. One radiologist evaluated all ultrasound images of each tumor on a monitor of the PACS system (StarPACS; Infinitt, Seoul, Korea) and then saved a minimum of two softcopy images from a transverse and a longitudinal plane of each tumor in the TIFF format. In patients with multiple breast cancers, only the largest lesion was considered. The images were labeled with the initials of each patient and with the date of birth. One experienced breast radiologist analyzed the saved electronic figures on a monitor. If a case was unclear, a second breast radiologist was consulted.
Tumor characteristics were assessed using the BI-RADS®-Ultrasound lexicon Citation[11] and an appropriate reference Citation[10]. The characteristics considered were shape (round or oval vs. irregular), margin (circumscribed vs. not circumscribed), orientation (parallel vs. not parallel to skin), boundary (abrupt interface vs. echogenic halo), echo pattern (hypoechoic or complex vs. isoechoic or hyperechoic), posterior acoustic feature (shadowing or combined posterior acoustic feature vs. no posterior acoustic feature or enhancement), and the presence of calcifications within a mass. “Not circumscribed” margins were defined when the margin was indistinct, spiculated, angular, or microlobulated.
Histological examination
The breast specimens were formalin-fixed, paraffin-embedded tissue blocks subsequently stained with hematoxylin and eosin. Histological tumor types were divided into invasive cancers and ductal carcinoma in situ (DCIS). Invasive cancer was graded as grade 1 (well differentiated), grade 2 (moderately differentiated), or grade 3 (poorly differentiated) according to the Scarff-Bloom-Richardson System Citation[12]. DCIS cases were classified as group 1 (nonhigh grade DCIS without comedo-type necrosis), group 2 (nonhigh grade DCIS with comedo-type necrosis), or group 3 (high grade DCIS with or without comedo-type necrosis) according to the Van Nuys Classification Citation[13].
Formalin-fixed, paraffin-embedded tissue sections were stained by immunohistochemical with appropriate antibodies for (a) ER (antibody SP1, Neomarker, Fremont, CA, USA), dilution 1:100, 30 minutes incubation at room temperature; (b) PR (antibody PgR 636, DAKO, Carpinteria, CA USA), dilution 1:20, 30 minutes incubation at room temperature; and (c) HER-2/neu (antibody TAB250, Zymed, San Francisco, CA USA), dilution 1:200, 1 hour incubation at room temperature. ER and PR were scored positive Citation[14], Citation[15] if more than 10% or tumor cells were immunoreactive by evaluation of 10 random microscopic fields comprising at least 1 000 tumor cells. HER-2/neu status was graded as 0, 1+, 2+, and 3+, and 3+ was determined as positive Citation[16].
Statistical analysis
For correlations we performed univariate logistic regression models and expressed the odds ratio (OR) with 95% confidence intervals (CI), and also performed multivariate regression analysis. P-values lower than 0.05 were considered statistically significant. We also correlated five biological markers with tumor type and grade on a histological examination using the χ2 test. Analyses of the data were determined by a statistician using statistical software (SAS/STAT software, version 6.12; SAS Institute, Cary, NC USA).
Results
Biological markers correlated with the histological grade in invasive cancers (). ER negativity, PR negativity, and HER-2/neu positivity were more frequent in grade 3 invasive cancers than in grade 2/grade 1 invasive cancers (p<0.0001). There was no significant difference between invasive cancers and DCIS for the presence of the biological markers (p > 0.05).
Table II. Correlation of the biological markers with tumor type and tumor grade.
Results of the univariate and multivariate regression models comparing the ultrasound findings of the 458 breast cancers are presented in –VII. Differences were seen in shape, orientation, and echo pattern between the invasive cancers and DCIS (). An irregular shape (72% vs. 35%), a not parallel orientation (42% vs. 9%), and a hypoechoic or complex echo pattern (92% vs. 6%) were more frequent in invasive cancers when compared with the DCIS cases.
Table III. Tumor type and ultrasound findings of the breast cancers.
demonstrates a correlation of the ultrasound findings with tumor grades of the invasive cancers. There were significant differences in margin, boundary, and echo pattern between grade 3 and grade 2/grade 1 invasive cancers by multivariate regression analysis (p < 0.05). Not circumscribed margins (90% vs. 87%) (), an abrupt boundary (57% vs. 43%) ( and ), and a hypoechoic or complex echo pattern (95% vs. 91%) () were more frequent in grade 3 invasive cancers than in grade 2/grade 1 invasive cancers.
Figure 1. A 37-year-old female with a grade 3 invasive ductal carcinoma. An ultrasound image demonstrates an irregular shaped, not circumscribed marginated, isoechoic mass (arrows) with an abrupt boundary and internal calcifications (arrowheads). On a histological examination, the cancer was ER and PR positive and HER-2/neu positive.
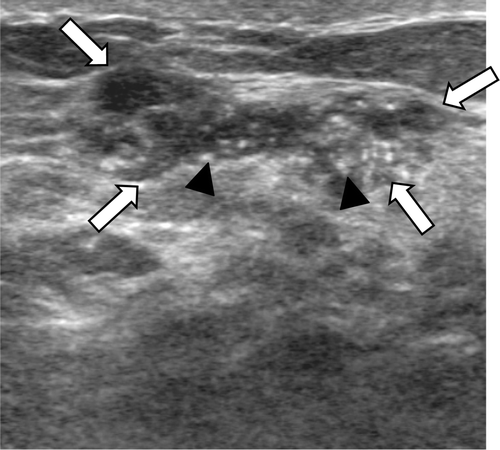
Figure 2. A 42-year-old female with a grade 3 invasive ductal carcinoma. Ultrasound shows an irregular shaped, circumscribed marginated, hypoechoic, parallel orientated mass (arrows) with an abrupt boundary and no internal calcifications. The cancer was ER and PR negative, and HER-2/neu negative.
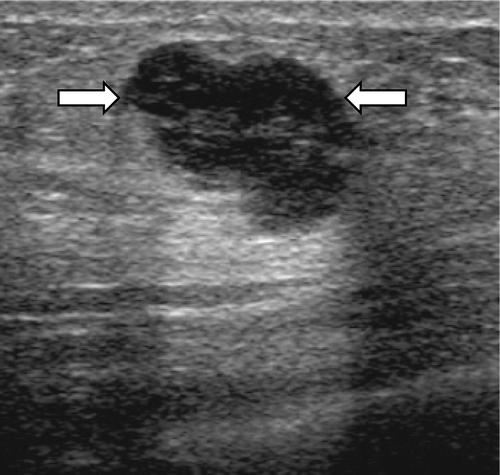
Table IV. Tumor grade and ultrasound findings of invasive breast cancers.
In the 23 DCIS cases, tumor classification was correlated with the presence of calcifications on ultrasound. Calcifications were more frequent in group 3 DCIS cases (10/13, 69%) than in group 2/group 1 DCIS cases (1/10, 10%) (p < 0.05).
A hypoechoic or complex echo pattern was more frequent in ER or PR negative cancers (96% in ER negative cancers, 94% in PR negative cancers) when compared with ER or PR positive cancers (87% in ER negative cancers, 88% in PR negative cancers) ( and ) (). HER-2/neu positivity correlated with the presence of calcifications (46% vs. 19%, p < 0.0001) () ().
Figure 3. A 44-year-old female with a grade 2 invasive ductal carcinoma. Ultrasound shows an irregular shaped, not circumscribed marginated, hypoechoic, not parallel orientated mass (arrows) with an echogenic halo boundary. The cancer was ER and PR positive, and HER-2/neu negative.
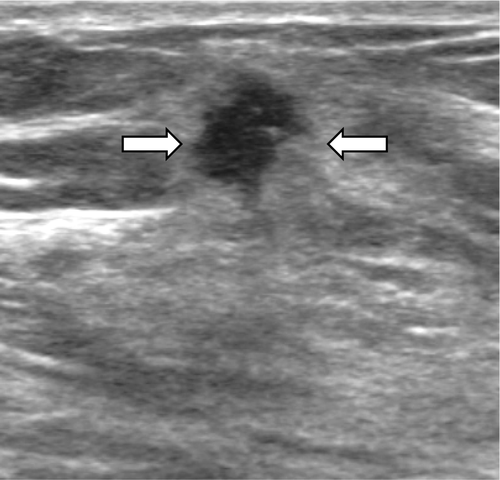
Table V. Estrogen receptor and ultrasound findings of the breast cancers.
Table VI. Progesterone receptor and ultrasound findings of the breast cancers.
Table VII. HER-2/neu oncogene and ultrasound findings of the breast cancers.
Discussion
The major role of breast ultrasound is to diagnose early breast cancers. Ultrasound can differentiate benign and malignant breast lesions and detect occult breast cancers in dense breasts Citation[7–10]. The goal of this study was to determine any correlation between ultrasound findings and prognostic indicators for breast cancers. Multiple logistic regression models demonstrated that tumor type, tumor grade, and the presence of biological markers had a significant impact on the ultrasound findings. Invasive cancers had more frequent breast cancers with an irregular shape, a not parallel orientation and a hypoechoic or complex echo pattern when compared with DCIS cases. These results are comparable to previous studies Citation[17], Citation[18]. An irregular shape, a not parallel orientation, and hypoechoic or complex echo pattern are the typical malignant features of solid breast masses Citation[10]. DCIS cases often have less of these typically malignant features; thus, radiologists or sonographers may misinterpret the lesion as being benign. Chen et al. Citation[17] have reported that the internal echo pattern was the most significant factor to differentiate invasive cancers and DCIS cases. Correlations of tumor grade and ultrasound findings in previous studies have varied. Lamb et al. Citation[19] described that high grade invasive cancers were more likely to demonstrate posterior acoustic enhancement and a well defined margin on ultrasound. Watermann and colleagues Citation[20] reported that tumor grading showed no correlation with the examined ultrasound criteria. In our study, the tumor grade of invasive cancers influenced the ultrasound findings. Our results revealed that a not circumscribed margin, an abrupt boundary, and a hypoechoic or complex echo pattern were more frequent in grade 3 than in grade 1–2 invasive cancers. Calcifications on ultrasound were more frequent in group 3 DCIS than group 1-2DCIS. However, the number of DCIS cases was few, 23. The most common feature of DCIS on mammography is microcalcifications. Linear branching or pleomorphic microcalcifications have a high predictive value for the high grade comedo type DCIS Citation[21].
ER, PR, and HER-2/neu expression has prognostic and therapeutic value in breast cancer. Our results revealed that all three biological markers correlated with the ultrasound findings. ER and PR demonstrated a significant correlation with echo pattern and a hypoechoic or complex echo pattern was seen more often in cancers with ER or PR negativity. Cancers with ER or PR positivity respond to hormonal therapy and have a relatively good prognosis. Thus, our findings suggest that an echo pattern noted on ultrasound is related with prognosis in breast cancer.
Assessment of HER-2/neu positivity is important for the establishment of a treatment plan and the prediction of prognosis in patients with primary breast cancer. HER-2/neu is a cell membrane receptor with growth-regulating activity and overexpression of HER-2/neu plays a direct role in oncogenic transformation. Overexpression of the HER-2/neu gene is associated with a number of adverse prognostic factors, including tumor size, axillary lymph node metastasis, hormone receptors, and tumor grade Citation[16]. In this study, expression of the HER-2/neu oncogene strongly correlated with presence of calcifications on ultrasound. A correlation between the overexpression of the HER-2/neu oncogene and calcifications was also reported by mammography in previous studies Citation[22], Citation[23]. Seo et al. Citation[22] described that fine linear morphology and diffuse distribution of calcifications on mammography were more frequent in breast cancers with HER-2/neu overexpression and the histological tumor grade in invasive cancers and that DCIS cases correlated with HER-2/neu overexpression. Thus, presence of calcifications on mammography or on ultrasound might be related to prognosis. One limitation of the HER-2/neu oncogene assessment in the current study was that we only used immunohistochemical methods. HER-2/neu status was graded as 0, 1+, 2+, and 3+, and only 3+ was determined as positive. A study using a large patient cohort by Yaziji et al. Citation[24] reported that for cancers with a weak positive immunohistochemical result, 17% of the 2+ staining cases were HER-2/neu positive by fluorescence in situ hybridization (FISH) Citation[24]. However, FISH was not available at our hospital.
Our results are based on a retrospective analysis. To avoid bias, we included all consecutive patients who underwent breast cancer surgery and preoperative breast ultrasound, and all examinations were reviewed by one experienced investigator who was blinded to the clinical data. In conclusion, findings on breast ultrasound correlate with the histological tumor type, tumor grade and biological markers in breast cancers and the use of breast ultrasound may be useful for predicting prognosis. Further studies are warranted with a large population to confirm our results.
Acknowledgements
This study was supported by the Korea University Grant.
References
- Clark G. Diseases of the Breast2nd edn. Lippingcott Williams & Wilkins, Philadelphia 2000
- Mouret-Reynier MA, Abrial CJ, Ferrière JP, Amat S, Curé HD, Kwaitkowski FG, et al. Neoadjuvant FEC 100 for operable breast cancer: 8-year experience at Centre Jean Perrin. Clin Breast Cancer 2004; 5: 303–7
- van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: A review. J Clin Pathol 2004; 57: 675–81
- Rosen PP. Rosen's Breast Pathology2nd edn. Lippingcott Williams & Wilkins, Philadelphia 2001
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004; 96: 739–49
- Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dünser M. Incidental findings on sonography of the breast: Clinical significance and diagnostic workup. AJR 1999; 173: 921–7
- Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. Cancer 1995; 76: 626–30
- Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: Detection with screening US-diagnostic yield and tumor characteristics. Radiology 1998; 207: 191–9
- Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR 2003; 181: 177–82
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology 1995; 196: 123–34
- American College of Radiology. Breast imaging reporting and data system (BI-RADS®)-Ultrasound. 1st ed. Reston: ©American College of Radiology; 2003.
- Bloom HJG, Richardson WW. Histologic grading and prognosis in breast cancer: A study of 1709 cases of which 359 have been followed for 15 years. Br J Cancer 1957; 11: 353–77
- Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet 1995; 345: 1154–7
- Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocystochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res 1990; 50: 7057–61
- Guerra I, Algorta J, Díaz de Otazu R, Pelayo A, Fariña J. Immunohistochemical prognostic index for breast cancer in young women. Mol Pathol 2003; 56: 323–7
- Taucher S, Rudas M, Mader RM, Gnant M, Dubsky P, Bachleitner T, et al. Do we need HER-2/neu testing for all patients with primary breast carcinoma?. Cancer 2003; 98: 2547–53
- Chen SC, Cheung YC, Lo YF, Chen MF, Hwang TL, Su CH, et al. Sonographic differentiation of invasive and intraductal carcinomas of the breast. Br J Radiol 2003; 76: 600–4
- Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics 2002; 22: 269–80
- Lamb PM, Perry NM, Vinnicombe SJ, Wells CA. Correlation between ultrasound characteristics, mammographic findings and histological grade in patients with invasive ductal carcinoma of breast. Clin Radiol 2000; 55: 40–4
- Watermann DO, Tempfer CB, Hefler LA, Parat C, Stickeler E. Ultrasound criteria for ductal invasive breast cancer are modified by age, tumor size, and axillary lymph node status. Breast Cancer Res Treat 2005; 89: 127–33
- Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: Mammographic-pathologic correlation. Semin Diagn Pathol 1994; 11: 181–92
- Seo BK, Pisano ED, Kuzimak CM, Koomen M, Pavic D, Lee Y, et al. Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol 2006; 13: 1211–8
- Karamouzis MV, Likaki-Karatza E, Ravazoula P, Badra FA, Koukouras D, Tzorakoleftherakis E, et al. Non-palpable breast carcinomas: Correlation of mammographically detected malignant-appearing microcalcifications and molecular prognostic factors. Int J Cancer 2002; 102: 86–90
- Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 2004; 291: 1972–7