To the Editor
Chronic myelogenous leukemia (CML) is a genetically heterogenous disease that is frequently associated with cytogenetic abnormalities Citation[1]. A second philadelphia (Ph) chromosome, trisomy 8, isochromosome 17q and trisomy 19 are the most common karyotypic abnormalities found in CML patients Citation[2]. Trisomy 14 is a rare chromosomal aberration that is usually observed in myelodysplastic syndrome, myeloproliferative disorders, atypical chronic myeloid leukemia and acute myeloid leukemia which is associated with old age, thrombocytosis and poor prognosis Citation[3–6]. Robertsonian translocations are rarely reported in hematological malignancies and the incidence is estimated to be 1/10.000 Citation[7]. However, trisomies resulted from Robertsonian translocations are extremely rare Citation[8]. In the literature there is only one CML patient diagnosed in blastic phase carrying both Ph chromosome and translocation (14;14) who has a similar disease progress as in our case Citation[9].
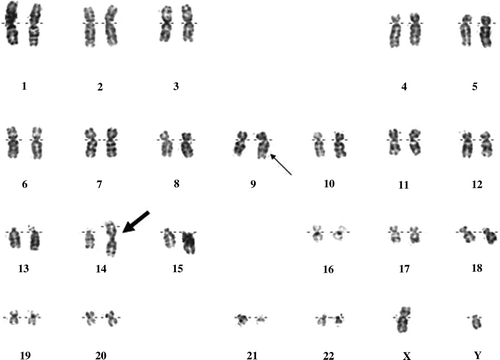
A 55-year-old man was admitted to our hospital with fatigue, weakness, weight loss, night sweating, abdominal pain, dry cough and fever of more than 39°C for 10 days. On physical examination, there was echymosis along the skin of the extremities, hepatomegaly and massive splenomegaly. The complete blood count showed white blood cells (WBC) 213×109/L, hemoglobin (Hb) 8.1 g/dl, hematocrit (Hct) 27.6% and platelets (PLT) 137×109/L. Biochemical parameters were within normal limits except lactate dehydrogenase (LDH) 1794 U/L. Peripheral blood smear (PS) revealed the leukocytosis and the presence of 60% of myeloloblastic cells where 3% of them were peroxidase positive. Bone marrow aspiration and biopsy specimen were consistent with the diagnosis of acute myeloblastic leukemia FAB myelomonocytic type (AML-FAB4) showing 60% of myelomonoblastic infiltration with CD34+, MPO+, Lyzozyme+, CD68+ and CD3−, CD79a−, TdT- by immunohistochemical staining. Broad spectrum antibiotherapy was started for the febrile status and cytarabine and idarubicine were given as a remission induction chemotherapy. One month after the chemotherapy, his hematological parameters improved to WBC: 3.1×109/L where 65% of them were neutrophils, Hb: 9.4 g/dl, Hct: 29.8%, PLT: 370×109/L. However, bone marrow aspiration showed 50% myeloblastic cell infiltration. After the salvage therapy with fludarabine plus cytarabine, a hematological remission was not achieved. At diagnosis, the karyotype obtained from unstimulated culture of bone marrow showed trisomy 14 due to Robertsonian translocation, a philadelphia chromosome and was reported as 46,XY,t(14;14)(q10;q10),t(9;22)(q34;q11.2) (). Fluorescent in situ hybridisation (FISH) analysis confirmed the Ph chromosome and the robertsonian translocation in metaphase chromosomes. Together with the philadelphia chromosome positivity, the diagnosis of the patient was considered as CML in blastic transformation and 600 mg/day imatinib therapy was given. Six months after the imatinib treatment, bcr-abl levels decreased from the first level of 0.448 to 0.0074 which was consistent with major molecular response; however there was persistence of 6% blastic cells in bone marrow. The control karyotype performed during this period was also consistent with trisomy 14 and a Ph chromosome. Another tyrosine kinase inhibitor, dasatinib was started and the control bone marrow biopsy after three months revealed 10% blastic cells. The case was considered as drug resistant CML and he does not have a suitable stem cell donor at the moment. Nilotinib therapy has been planned for the future treatment.
Figure 1. G-banded karyogram from bone marrow cells of the patient showing trisomy 14 due to a Robertsonian translocation, normal and derivative chromosomes 9 and 22. Karyotype of the patient was noted as 46,XY,t(14;14)(q10;q10),t(9;22)(q34;q11.2). The Robertsonian translocation is indicated by the thick arrow and the Ph chromosome was marked with the thin arrow.
Our case was considered to be a blastic crisis of CML by the presence of Ph positivity in all the metaphases obtained from the unstimulated culture of bone marrow at diagnosis and after chemotherapy. Although most of the patients are diagnosed during the chronic phase, less than 10% present with the accelerated or blastic phases as in the presented case Citation[10]. In the literature, there are very few reports about the presence of both Ph chromosome and trisomy 14 in CML. To our knowledge, this is the first case of CML having trisomy 14 due to a Robertsonian translocation. Interestingly a similar disease progress in a 68-year-old CML case with t(14;14)(q11;q32) and Ph chromosome who had B cell phenotype at diagnosis and a biphenotypic (lymphoid-myeloid) expression at relapse was reported Citation[10]. On the contrary of the previously reported case, no complete hematological remission was achieved in our case but major molecular response was obtained. In remission of the previously described case, t(14;14) disappeared but in our patient, trisomy 14 persisted during the molecular response period. Also, therapy with the new and effective tyrosine kinase inhibitors did not alter the prognosis in this patient. This clinical picture may be related to the total trisomy of the chromosome 14 and the Robertsonian translocation may also play a role in the poor prognosis. Further case reports will be needed to confirm these findings. This report reinforces the importance of conventional karyotyping in the diagnosis and prognosis of hematological malignancies and supports the theory that trisomy 14 is a rare cytogenetic abnormality found in myeloid malignancies and is associated with poor prognosis. This may lead strategies to further increase the understanding of drug resistant CML and may represent next frontier in the targeted therapy of CML patients.
References
- Ostro D, Cheung K, Kamel-Reid S, Lipton JH. Chromosomal abnormalities in chronic myeloid leukemia: Evidence of a hierarchy in imatinib treated cells. Leuk Lymphoma 2007; 48: 1029–31
- Orciuolo E, Buda G, Galimberti S, Sordi E, Cervetti G, Petrini M. Concomitant appearance of trisomy 8 and isochromosome 17q in a Philadelphia-positive clone in a patient with chronic myeloid leukemia in chronic phase: An alarm for changing therapeutic strategy. Cancer Genet Cytogenet 2007; 177: 166–7
- Toze CL, Barnett MJ, Naiman SC, Horsman DE. Trisomy 14 is a non-random karyotypic abnormality associated with myeloid malignancies. Br J Haematol 1997; 98: 177–85
- Reddy S. Trisomy 14 and leukemia. Cancer Genet Cytogenet 1998; 106: 144–51
- Vasef MA, Murata-Collins JL, Alsabeh R, Medeiros LJ. Trisomy 14 in myelodysplastic syndromes. Arch Pathol Lab Med 1998; 122: 77–83
- Horton YM, Johnson PR. Trisomy 14 in myeloid malignancies: Report of two cases and review of the literature. Cancer Genet Cytogenet 2001; 124: 172–4
- Hecht F, Morgan R, Hecht BK. Robertsonian chromosome recombinants are rare in cancer. Cancer Genet Cytogenet 1998; 35: 79–81
- Shimokawa T, Sakai M, Kojima Y, Takeyama H. Acute myelogeneous leukemia (M5a) that demonstrated chromosomal abnormality of robertsonian 13;21 translocation at onset. Intern Med 2004; 43: 508–11
- Dastugue N, Kuhlein E, Duchayne E, Roubinet F, Bourrouillou G, Attal M, et al. t(14;14)(q11;q32) in biphenotypic blastic phase of chronic myeloid leukemia. Blood 1986; 68: 949–53
- Bacher U, Haferlach T, Hiddemann W, Schnittger S, Kern W, Schoch C. Additional clonal abnormalities in Philadelphia-positive ALL and CML demonstrate a different cytogenetic pattern at diagnosis and follow different pathways at progression. Cancer Genet Cytogenet 2005; 157: 53–61
