Abstract
Background. About 2% of patients with a carcinoma in one kidney develop either metastases or a new primary tumor in the contralateral kidney. Often, renal cancers progress rapidly at peripheral sites and a metastasis to the second kidney may not be the patient's main problem. However, when an initial renal cancer is more indolent yet spreads to the formerly unaffected kidney or a new primary tumor forms there, local treatment may be needed. Stereotactic body radiotherapy (SBRT) has been demonstrated as a valuable treatment option for tumors that cause local symptoms. Presented here is a retrospective analysis of patients in whom SBRT was used to control primary or metastatic renal disease. Patients and methods. Seven patients with a mean age of 64 (44–76) were treated for metastases from a malignant kidney to its contralateral counterpart. Dose/fractionation schedules varied between 10 Gy×3 and 10 Gy×4 depending on target location and size, given within one week. Follow-up times for patients who remained alive were 12, 52 and 66 months and for those who subsequently died were 10, 16, 49 and 70 months. Results. Local control, defined as radiologically stable disease or partial/complete response, was obtained in six of these seven patients and regained after retreatment in the one patient whose lesion progressed. Side effects were generally mild, and in five of the seven patients, kidney function remained unaffected after treatment. In two patients, the creatinine levels remained moderately elevated at approximately 160 µmol/L post treatment. At no time was dialysis required. Conclusion. These results indicate that SBRT is a valuable alternative to surgery and other options for patients with metastases from a cancer-bearing kidney to the remaining kidney and provides local tumor control with satisfactory kidney function.
Renal cancer affects about 60 000 persons in the EU every year Citation[1]. For those with advanced renal cell carcinoma, the therapeutic options are limited. Systemic therapy was, for many years limited to interleukin-2 and interferon alpha (IFN-α), but new agents like sunitinib and sorafinib have now imparted significantly improved progression free survival times Citation[2], Citation[3]. However, patients’ responses are not assured, and the treatments are not curative. Moreover, systemic therapy may be inadequate for patients whose local metastases pose a threat to the functions of their vital organs, especially individuals with few metastatic lesions and an indolent disease. About 2% of patients with a carcinoma in one kidney develop additional primary tumors or metastases to the formerly unaffected, second kidney Citation[4], and some patients with, for instance, von Hippel-Lindau disease or a family history of renal cancer, have a high risk of bilateral involvement Citation[5].
The option of nephrectomy, when technically possible for patients with a sole functioning kidney, requires careful consideration, but sometimes seems to be the only choice Citation[6–8]. A study comparing survival rate after surgery to that without intervention revealed a significant 67% post-operative survival compared to only 17% of the untreated group after three years Citation[9]. Reports in the literature of initial renal cell carcinomas metastasizing to the second kidney have shown that the disease is usually located in the renal poles Citation[10] allowing such nephron-sparing surgical techniques as enucleation, partial nephrectomy, cryoablation, implantation of seeds or radioablation Citation[11–14]. Nephron-sparing surgery has from an efficacy point of view similar results as nephrectomy Citation[15]. Still, since surgery is not suitable for all patients due to poor performance status, there is a need for less or non- invasive methods as treatment alternatives.
Now, stereotactic body radiation therapy (SBRT) has yielded a high rate of local control for primary tumors and metastases in numerous tumor types including those of the kidneys Citation[16–21]. Therefore, SBRT shows potential as an alternative to surgery, which may be technically difficult and counter-indicated for patients with poor performance status from oligo-metastatic disease. This report recounts our function-preserving experience with SBRT for seven patients with primary tumors or metastases in a second kidney.
Patients and methods
Patients
The patient population consisted of six women and one man with a mean age of 64 years (range 44–76), who underwent SBRT for lesions in a second cancer-affected kidney between 1997 and 2005 (). This group was part of a larger, previously described series of 58 patients with renal cell carcinomas Citation[20]. All patients had a previous nephrectomy and a Karnofsky index of 60 or higher. Six patients had indolent metastatic disease with multiple metastases. Only one of them had a risk factor for worse prognosis, according to Motzer criteria Citation[22]. Because of distant metastases, two patients received treatment with IFN-α or IFN-α + interleukin-2 prior to SBRT, with transient effect. One patient who lost her first kidney due to multiple infections and malformation during childhood was treated for a primary tumor in her remaining kidney. Later, she underwent retreatment due to local progression after the first SBRT session. Six patients were treated for metastases to a second kidney and also received SBRT to other lesions. Another patient received IFN-α after SBRT, and another had intravenous injections of autologous dendritic cells, without any effect. All patients had normal kidney function before treatment. An overview of their treatments appears in .
Table I. Clinical summary of renal cell carcinomas.
In a separate cohort of control patients without kidney disease, the renal volume was measured after CT, using the dose planning program (TMS, Helax). Nine were women and six were men. Of their 27 kidneys evaluated, 12 were left sided and 15 right sided.
SBRT
The SBRT method has been described Citation[18]. In short, this method's basic components include 1) a stereotactic body frame for localization of the target and set-up at the treatment unit, 2) CT for geometrical verification of the tumor position in the stereotactic system, 3) a planned heterogeneous dose distribution within the planning target volume (PTV) and 4) hypofractionation schedules with three or four fractions given every second day. Furthermore, to minimize the movement of the target with respiration, an abdominal pressure device was used that reduced diaphragm motions to within±5 mm.
A clinical target volume (CTV) was defined on CT scans. In most cases it was identical with the gross tumor volume (GTV). Around the CTV, a margin of 5 to 10 mm was added in the transverse and 10 mm in the cranio-caudal directions to obtain the PTV. The remaining healthy kidney parenchyma, i.e., excluding the CTV, was defined as a separate volume, here called organ-at-risk (OAR-kidney). Dose-volume histograms (doses converted to 2 Gy/fraction (α|β = 3) for each dose step) and the mean doses were calculated for this volume. As a further measure of the dose distribution within the OAR-kidney, the effective volume was calculated (Veff), i.e., the OAR-kidney volume, which if irradiated uniformly with the prescribed dose, would be associated with the same normal tissue complication probability as the non-uniform dose distribution actually delivered Citation[23]. In this calculation, the parameter “n” was assumed to be 0.97. We also calculated the V15, i.e. the relative volume of OAR-kidney that received 15 Gy or more. For the most common fractionation in this study, 10 Gy×3, V15 corresponds to the volume receiving the 50% isodose (EQD2=24 Gy) or higher.
Treatment technique and dose distribution
The SBRT used in the present study was a conformal technique that uses five to eight coplanar or non-coplanar static beams, as previously described Citation[18], Citation[24]. The directions of the beams were spread in a large solid angle, taking into account the locations of OARs. The beams were shaped by a multileaf collimator to conform to the target. A photon energy of 6 MV was used. For dose planning, the pencil beam algorithm in the Helax-TMS system was used. The slice thickness of CT images was 5 mm.
Fractionation
Several factors were considered when deciding the dose/fraction, number of fractions and interval between irradiations. These included size of the CTV, dose distributions with dose-volume histograms and radiation sensitivity of surrounding normal tissues. Usually OARs for these patients were the treated kidney itself, sometimes the gut and to a certain extent the medulla. We tried to spare as much healthy kidney as possible. The volumes of OAR-kidney are shown in . The locations of the treated tumors in the kidneys are shown in (). To avoid complications in the gut, we did not allow doses higher than 7 Gy/fraction to that site. Doses for the medulla were kept below 5 Gy/fraction in all patients, because their renal tumors were rather small. Treatments of three or four 10 Gy/fraction were applied to the periphery of the PTV and given every second day. The patient with a local treatment failure received 10 Gy×3 as an initial treatment and 10 Gy×4 in the re-treatment session.
Figure 1. Approximate size and location in the kidneys of all tumors treated. Note that all patients had only one kidney at SBRT. This summation pictures what a projection of the treated tumor looks like in a normal individual.
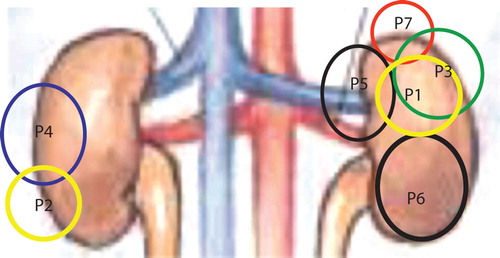
Table II. Data for patients treated for metastases of an initial renal cancer to a second kidney.
Post-treatment follow-up
Clinical examinations with blood chemistry and CT scans were performed at 3-month intervals following SBRT. Blood factors including creatinine were analyzed monthly for six months. Intervals between clinical examinations were extended to six months in patients without recurrences or with stable disease for more than two years. Maximum FU-time was 70 months in patient no 7. Censor date of this study was December 31, 2006.
Results
Of the seven patients described here, three patients were still alive at censor date and their total follow-up times were 12, 52 and 66 months after SBRT. The four patients who died had been followed for 10, 16, 49 and 70 months.
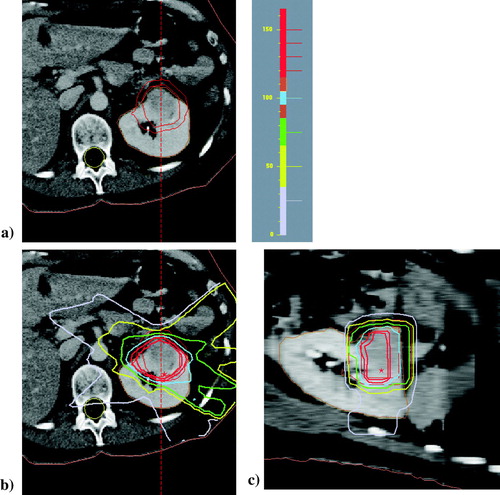
So far, local recurrence has been observed in one patient, leading to retreatment after 54 months (). The second follow-up period is, so far, 12 months with no new, local recurrence.
Figure 2. Upper figure (a) shows the CTV and PTV of patient 5 at first treatment. Lower figures (b) shows the relative dose distribution, with the prescription isodose (100% in blue), at the periphery of the PTV.c) Relative dose distribution to the target in sagittal view. The color codes of the isodoses are also shown.
All patients had normal creatinine levels before SBRT () and, in all but two, the serum creatinine remained at that level after treatment. One patient's serum creatinine increased from ∼120 µmol/L to 150–160 µmol/L after two years of follow-up and has stabilized at this level up to the latest visit after 52 months (pat no. 4).
Figure 3. Serum creatinine levels in all seven patients during follow-up. (.-◊-.) patient 1, (- -▴ - -) patient 2, (….∇….) patient 3, (- -▪- -) patient 4, (–•–) patient 5, (–○–) patient 6, (-.-•-.-) patient 7.
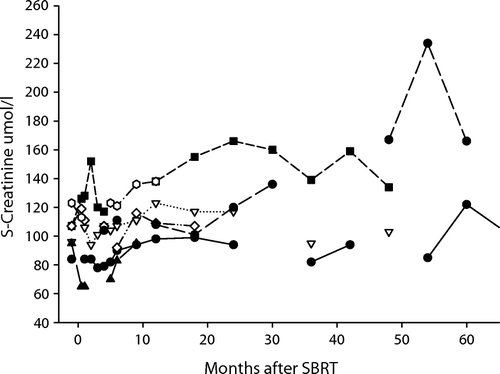
Patient no. 5 has had just one kidney since childhood. After receiving SBRT twice in that kidney, her creatinine levels have increased only slightly, about 20% (85–104 µmol/L), during six years of follow-up. A recent GFR-value (glomerular filtration rate) measured with iohexol clearance showed a value of 56 ml/min (corrected to her body size).
None of the patients needed dialysis. Transient side effects grade I-II (nausea, fatigue and local pain) were observed in four of the patients.
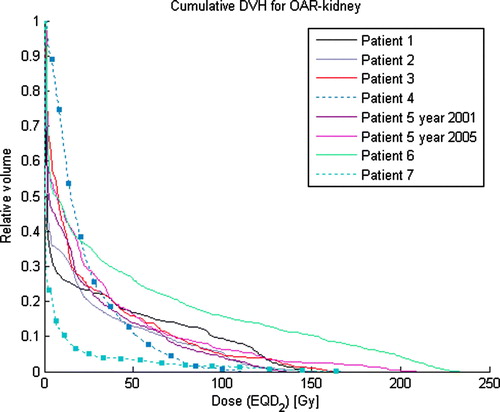
For comparison, renal volumes were measured in 15 patients (27 kidneys) without renal disease. The mean volume was 171±41 cm3 (SD), and the median was 170 (range 102–270 cm3). The mean volume of kidney parenchyma was 153 cm3 for women and 193 cm3 for men, 180 cm3 for left kidneys and 164 cm3 for right kidneys. In our patients with renal cancer evaluated here, the mean value of the second kidney's volume was 241±64 cm3 (SD), and the median was 221 (177–359) cm3. The dose-volume histograms for the parenchyma (OAR-kidney) are shown in . Mean doses and effective volumes of OAR-kidneys appear in .
Figure 4. Cumulative dose-volume histogram for OAR-kidneys in all seven patients. Dose on the x-axis is expressed as equivalent dose in 2 Gy/ fraction (EQD2), and volume on the y-axis as relative volume (percent of remaining normal kidney parenchyma minus CTV). Patients no. 4 and 7 are marked with filled boxes.
Discussion
The results reported here of seven patients with renal cell carcinomas whose disease was monitored for over six years show that the use of SBRT for patients with cancer lesions in their only functional kidney yields a high degree of local control with no negative effect on that function.
An important question in the treatment of metastatic lesions in solitary kidneys, regardless of the technique used, is how much of the organ's volume should be spared to avoid kidney failure. In animal models (mainly rats), nephrectomies have been performed leaving only up to 1/3 of a kidney intact. The condition called “overload-nephropathy” followed, i.e., the majority of these animals developed uremia. Therefore, the remaining 15 to 17% of normal kidney tissue is, apparently, too little for acceptable function over a sustained period of time. From clinical experience, we know that the lowest glomerular filtration rate with which renal function remains stable is about 20 to 25 ml/min. This rate can be maintained by roughly one-half of a kidney. Of course, individuals vary in this respect, and glomerular filtration rates also decrease over time to approximately 20% lower values after the age of 60 than in a healthy 20-year-old Citation[25].
In our patients, the second kidney to become cancerous had a larger volume than normally functioning kidneys in most individuals. Although the parenchyma in that second kidney also received some SBRT dosage, less than 40% of the tissue received doses >20 Gy (equivalent dose in 2 Gy fractions-EQD2), i.e., 60% of the remaining kidney volume received doses of <20 Gy with a dose per fraction of 2.0 Gy (). Because most information about kidney dose-responses is based on conventional fractionation, the available data is sparse. The endpoint in those studies has been nephropathy or clinical nephritis measured with varying methods, and follow-up was 3 to 8 years. In most studies, a “safe” dose to the whole kidney volume is considered to be 20 Gy in 2 Gy fractions, with less than a 5% complication rate in 5 years. Since the complication rate curve in normal tissue is very steep for the kidneys, even slightly greater doses of 25 to 30 Gy raise that rate to >50%, and doses above 40 Gy to the entire organ cause nephropathy in most patients Citation[26–28]. Scintigraphic changes without clinical symptoms can be observed at doses of <10 Gy to the whole kidney Citation[29]. Follow-up periods in these studies have not exceeded 8 years; therefore, caution is recommended for calculating doses for patients with longer expected survival times.
Here, the effective volume of the second kidney's parenchyma was calculated with the parameter n, which represents the volume effect, equal to 0.97, i.e., a large volume effect. This value has been shown to be valid for liver parenchyma Citation[30], but the value of n for kidneys is not well established. However, the data presented here clearly show that the kidney also has a very large volume effect. Furthermore, the effective volume calculated here has a moderately large dependence on n values in the range from 0.8 to 1.1. Interpretation of the effective volume shown in is that about one- quarter of the second kidney's parenchyma could be given the prescribed dose with the remaining part left unirradiated to achieve the same normal tissue complication probability as the actual dose distribution used.
Throughout this more than six year study, our patients were monitored for clinical status, electrolytes and serum creatinine levels. None of them developed hypertension after SBRT, and none needed dialysis. The only side effects noted were minor (grade I–II) and included transient nausea, fatigue and local pain in four patients.
In summary, these pilot cases demonstrate that SBRT may be an alternative to surgery or other therapeutic options for patients with primary or metastatic lesions in the only functional kidney remaining after the onset of a renal cell carcinoma. Correctly used, this technique provides a high degree of local control with mild side effects and enables that kidney to retain adequate function.
Acknowledgements
This research was supported by Cancerfonden.
References
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; 16: 481–8
- Motzer RJ, Hutson TE, Tomczak P, Michaelsson MD, Bukowski RM, Rixe O, et al. Sutinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–24
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356: 125–34
- Bani-Hani AH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML. Associations with contralateral recurrence following nephrectomy for renal cell carcinoma using a cohort of 2,352 patients. J Urol 2005; 173: 391–4
- Malek RS, Omess PJ, Benson RC, Jr, Zincke H. Renal cell carcinoma in von Hippel-Lindau syndrome. Am J Med 1987; 82: 236
- Grimaldi G, Reuter V, Russo P. Bilateral non-familial renal cell carcinoma. Ann Surg Oncol 1998; 5: 548
- Stephens R, Graham SD, Jr. Enucleation of tumor versus partial nephrectomy as conservative treatment of renal cell carcinoma. Cancer 1990; 65: 2663
- Gohji K, Kamidono S, Yamanaka N. Renal carcinoma in a solitary kidney. Br J Urol 1990; 66: 248
- Novick AC, Stewart BH, Straffon RA, Banowsky LH. Partial nephrectomy in the treatment of renal adenocarcinoma. J Urol 1977; 118: 932
- Henriksson C, Geterud K, Aldenborg F, Zachrisson BF, Pettersson S. Bilateral asynchronous renal cell carcinoma. Computed tomography of the contralateral kidney 10–43 years after nephrectomy. Eur Urol 1992; 22: 209
- Bhayani SB, Allaf ME, Link RE, Rao P, Varkarakis JM, Jarrett TW, et al. Laparoscopic partial nephrectomy in patients with neoplasia in a solitary kidney. Urology 2004; 64: 35–7
- Marshall FF, Taxy JB, Fishman EK, Chang R. The feasibility of surgical enucleation for renal cell carcinoma. J Urol 1986; 135: 231
- Varkarakis IM, Allaf ME, Inagaki T, Bhayani SB, Chan DY, Su LM, et al. Percutaneous radio frequency ablation of renal masses: Results at a 2-year mean followup. J Urol 2005; 174: 456–60
- Qi DF, Wu KJ, Li X, Zeng GH, Chen WZ. Treating renal carcinoma with ablation or ablation combined with 125I seed implantation: A report of eleven cases. Ai Zheng 2005; 24: 587–90
- Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000; 75: 1236–42
- Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Gunven P, Blomgren H, Lax I. Radiosurgery for recurring liver metastases after hepatectomy. Hepatogastroenterology 2003; 50: 1201–4
- Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: A noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004; 60: 186–96
- Wersall PJ, Blomgren H, Lax I, Kalkner KM, Linder C, Lundell G, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005; 77: 88–95
- Svedman C, Sandström P, Pisa P, Blomgren H, Lax I, Kälkner K-M, et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006; 45: 870–5
- Schwartz LH, Mazumdar M, Wang L, Smith A, Marion S, Panicec DM, et al. Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer 2003; 98: 1611–9
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume model. Int J Radiat Oncol Biol Phys 1989; 16: 1623–30
- Lax I, Blomgren H, Larson D, Näslund I. Extracranial stereotactic radiosurgery of localized targets. J Radiosurgery 1998; 1: 135–48
- Lalich JJ, Faith GC, Harding GE. Protein overload nephropathy in rats subjected to unilateral nephrectomy. Arch Pathol 1970; 89: 548–59
- Kim TH, Sommerville PJ, Freeman CR. Unilateral radiation nephropathy the long-term significance. Int J Radiat Oncol Biol Phys 1984; 10: 2053–9
- Dewit L, Anninga JK, Hoefnagel CA, Nooijen WJ. Radiation injury in the human kidney: A prospective analysis using specific scintigraphic and biochemical endpoints. Int J Radiat Oncol Biol Phys 1990; 19: 977–83
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–22
- Köst S, Dörr W, Keinert K, Glaser F-H, Endert G, Herrmann T. Effect of dose and dose-distribution in damage to the kidney following abdominal radiotherapy. Int J Radiat Biol 2002; 78: 695–702
- Bani-Hani AH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML. Associations with contralateral recurrence following nephrectomy for renal cell carcinoma using a cohort of 2,352 patients. J Urol 2005; 173: 391–4