Abstract
Background and purpose. As techniques for radiotherapy delivery have developed, increasingly accurate localisation of disease is demanded. Functional imaging, particularly PET and its fusion with anatomical modalities, such as PET/CT, promises to improve detection and characterisation of disease. This study evaluated the impact of 18FDG-PET/CT on radiotherapy target volume definition in head and neck cancer (HNC). Materials and methods. The PET/CT scans of patients with HNC were used in a radiotherapy planning (RTP) study. The gross tumour volume (GTV), clinical target volume (CTV) and planning target volume (PTV) were defined conventionally and compared to those defined using the PET/CT. Data were reported as the median value with 95% confidence intervals. Results. Eighteen patients were consented, 9 had known primary tumour site, 9 presented as unknown primary. In nine cases where the primary site was known, the combined primary and nodal GTV (GTVp+n) increased by a median of 6.1cm3 (2.6, 12.2) or 78% (18, 313), p=0.008 with CTV increasing by a median of 10.1cm3 (1.3, 30.6) or 4% (0, 13) p=0.012. In 9 cases of unknown primary the GTVp+n increased by a median 6.3cm3 (0.2, 15.7) or 61% (4, 210), p=0.012, with CTV increasing by a median 155.4cm3 (2.7, 281.7) or 95% (1, 137), p=0.008. Conclusion. 18FDG-PET revealed disease lying outside the conventional target volume, either extending a known area or highlighting a previously unknown area of disease, including the primary tumour in 5 cases. We recommend PET/CT in the RTP of all cases of unknown primary. In patients with a known primary, although the change in volume was statistically significant the clinical impact is less clear. 18FDG-PET can also show areas within the conventional target volume that are hypermetabolic which may be possible biological target volumes for dose escalation studies in the future.
As techniques for more accurate radiotherapy delivery to head and neck tumours such as intensity modulated radiotherapy (IMRT) have developed, the technology for accurate localisation of disease has lagged behind. CT and MRI have established roles in the diagnosis and staging of HNC and are routinely used in radiotherapy planning in this region of the body. However, there are limitations in anatomical imaging modalities that require a change in morphology to indicate abnormality. Small tumour deposits or metastases in normal sized lymph nodes cannot be identified. Treatment, either surgery or radiotherapy, in the head and neck region frequently results in altered anatomy or changed tissue planes, making interpretation of these images extremely difficult. The integration of functional imaging modalities into radiotherapy planning is gaining acceptance. The hope is that this will lead to improved staging of disease and reduction of geographical miss of tumour. Functional imaging modalities also promise to provide biological information specific to the tumour allowing image-guided treatment for individual phenotypes or biological target volumes (BTVs).
Combined functional and anatomical imaging modalities such as PET/CT have improved localisation of disease by providing spatial, anatomical mapping of biological processes. The fused images of 18FDG-PET/CT have been shown to improve diagnostic accuracy and to reduce inter-observer variation in head and neck cancer (HNC) Citation[1–3].
This study examined the impact of 18FDG-PET/CT on radiotherapy target volume definition in a group of patients presenting with HNC.
Methodology
Patients with newly-diagnosed carcinoma of the nasopharynx, oral cavity, oropharynx, hypopharynx or larynx or those who presented with cervical node metastases and no primary tumour, and had had a 18FDG-PET/CT scan as part of their work-up were asked if they would consent to the use of their images for this study.
This study was approved by the local ethical committee for our institution.
PET/CT
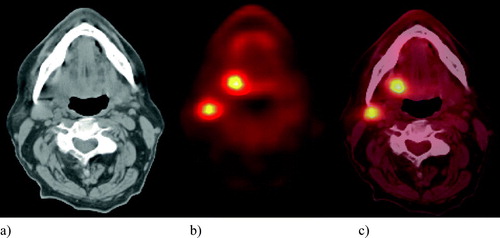
Patients were given verbal and written information about the scan procedure prior to their appointment. Diazepam (5 mg) was given by mouth 30 minutes prior to the 18FDG to reduce muscle and brown fat activity in the neck which is known to cause false positive uptake on 18FDG-PET. After a period of approximately 15 minutes (to reduce muscle tension), 370MBq of 18FDG was administered intravenously. Patients then lay quietly in a warm room for one hour prior to the scan. Patients emptied their bladder immediately before the scan. They were positioned supine head first on the scanner couch with their arms by their side and a tape fixed across the forehead to the couch top to aid immobilisation during the CT and PET parts of the acquisition and to limit movement between the scans to minimize misregistration of the PET and CT data. All patients had PET/CT scans () carried out on a PET/CT BiographTM LSO scanner (Siemens, Forscheim, Germany). No contrast medium was used for the CT scan. The following CT parameters were used: 130kV, 70mAs, reconstructed slice thickness 2.4 mm.
Conventional target volume definition using CT
GTV
Target volumes were defined according to the guidelines of ICRU Report No. 62 Citation[4] by a radiation oncologist (KN). Macroscopic disease identified on the CT defined the gross tumour volume (GTV). This macroscopic disease was identified by both KN and a consultant radiologist (BS). Findings were available from clinical examination including direct visual inspection, flexible nasendoscopy and examination under anaesthesia (EUA) if this had been performed. Information from radiological investigations such as CT and MRI was used to aid target delineation. The GTV of the primary tumour (GTVp) and of the nodal disease (GTVn) was outlined. A summed total GTV (GTVp + n) was also recorded.
CTV
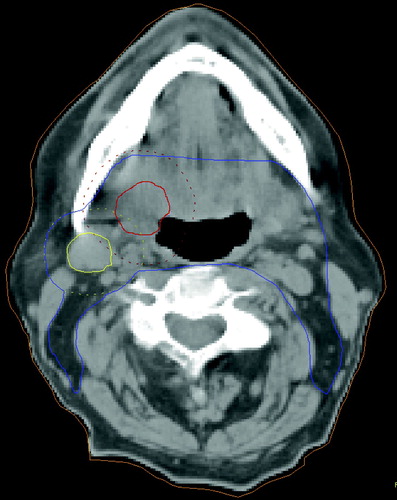
The clinical target volume (CTV) was defined as the GTV plus areas thought to be at risk of containing microscopic disease. The GTV of the primary and the involved nodes was grown by a margin of 1 cm and then expanded to include the whole of any nodal levels involved to create the high dose CTV. Nodal levels were outlined according to the consensus guidelines Citation[5]. Where the involved nodes suggested invasion into adjacent structures, e.g. muscle, these structures were also included in the CTV. For the primary tumour the entire organ, for example the oropharynx, was included (). For the purposes of this study the CTV was defined as that volume taken to a minimum of 50 Gy, i.e. to include both therapeutic and elective volumes.
Figure 2. Conventional target volume definition. Gross Tumour Volume of primary (GTVp), Gross Tumour Volume of nodal disease (GTVn), margin for microscopic spread added and entire organ outlined to form Clinical Target Volume (CTV). Labels: Red: GTVp (+1cm margin) Yellow: GTVn (+1cm margin). Blue: CTV.
PTV
The planning target volume (PTV) was defined by a geometric growth of the CTV by 3 mm. This margin has been defined within our department according to measured set-up error Citation[6].
Experimental target volume definition with PET alone
As with the conventional methods, target volumes were defined according to the guidelines of ICRU Report No. 62 Citation[4] by a radiation oncologist (KN).
Experimental GTV defined by PET alone
On the co-registered image, the PET image was set at 100% opacity so that the PET image only could be seen. Using the PET/CT diagnostic report issued by the consultant in nuclear medicine (GC) and consultant radiologist (BS), who were also available to review the images, the pathological region of interest (ROI) was identified. The PET GTV was then defined as that volume with a count density of 50% of the maximum of that particular region of interest. If the 50% level did not result in differentiation between pathological and physiological FDG uptake then a ‘pragmatic’ level was found subjectively with the guidance of the PET/CT consultants (GC and BS).
Experimental CTV defined by PET alone
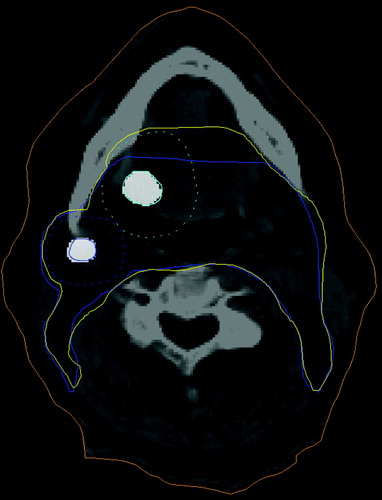
The translucency of the PET image was then set to 50% so the CT image was also visible. The PET-defined GTV was grown by a margin of 1cm to form the CTV then expanded in the same manner as for the conventional technique, using the CT image to include structures at risk and nodal levels. The CT-defined CTV was overlaid and used as a template in order to minimise intra-observer error in nodal level determination ().
Figure 3. Definition of the PET Clinical Target Volume. PET defined Gross Tumour Volume expanded with margin for microscopic spread and Conventionally defined CTV used as template to minimise intra-observer error. Labels: Cyan dashed: PET GTV primary expanded. Blue dashed: PET GTV node expanded. Blue: CT CTV. Yellow: PET CTV.
Experimental PTV defined by PET alone
The PTV was a geometrical expansion of the CTV by 3 mm as in the conventional method.
Experimental target volume definition with PET/CT
Finally, to obtain the co-registered PET/CT target volumes, the union of volumes of both modalities were used. That is to say where the conventionally defined GTV differed from the PET defined GTV, the PET/CT target volumes were defined by including both volumes, creating a composite volume.
Pathological TNM staging
The surgical and histopathology reports were examined to obtain a final TNM stage. The primary stage was obtained from the surgeons’ description and biopsy results either at the time of the neck dissection or at EUA. The nodal staging was derived from the pathological report of the neck dissection or the results of FNA or biopsy of the nodes in the absence of formal neck dissection.
Statistical analysis
The null hypothesis that the radiation target volumes defined by CT alone versus PET/CT would not be different was tested using the paired Wilcoxon sign rank test. P-values < 0.05 were considered to be statistically significant. Data are reported as the median value with 95% confidence intervals (CI).
Results
Eighteen patients were consented for the study, 9 had known primary tumour sites, 9 presented as unknown primaries. The latter group had all undergone clinical examination and in 5 cases examination under anaesthetic (EUA) with or without biopsy and radiological work up with chest radiograph, CT and/or MRI scan. All were male with a median age at the time of the scan of 52.5 (range 34-76) years. illustrates the characteristics of each case.
Table I. Case Characteristics, effect of the PET/CT on AJCC TNM staging, correlation with pathological stage and the effect of PET/CT on Clinical Target Volume (CTV)
The effect of the PET/CT scans on TNM staging and pathological correlation
The impact of the PET/CT scan on the AJCC TNM stage is shown in . The stage was changed in eight cases, all up-staged. In case 8, a clinically apparent primary in the left tonsil was not highlighted on the PET/CT but was confirmed by the pathology, however uptake was seen in the base of tongue which was also proven on pathology but had not been seen clinically. The PET/CT identified a primary site of disease in 5/9 (55.6%) of the cases presenting as unknown primaries. Three arose from the tonsil, two of these had not had EUAs and one had had an EUA but not a tonsillectomy. Two primaries were identified as arising from the tongue base, one had had an EUA and blind biopsies but these had not shown malignancy. The PET/CT-defined nodal staging was unchanged by TNM criteria but it is noteworthy that the PET/CT highlighted a contralateral node in case 14, although the overall stage remained at N3 on size criteria. The pathological staging confirmed the PET/CT stage in 17 cases, although neck dissection was not performed in cases 10 and 16, so more extensive nodal involvement could not be excluded, . Case 14 was downstaged by the pathology because contralateral nodal disease was not confirmed.
The effect of PET/CT on GTV
The combined primary and nodal GTV (GTVp + n) was increased by a median of 6.2 cm3 (4.2, 12.2) or 74% (38, 175). In the nine cases where the primary site was known, the GTVp + n increased by a median of 6.1 cm3 (2.6, 12.2) or 78% (18, 313), p = 0.008. In the 9 cases of unknown primary the GTVp + n increased by a median 6.3 cm3 (0.2, 15.7) or 61% (4, 210), p = 0.012. Out of these 9, in the 5 cases where the primary tumour was identified, GTVp + n increased by a median of 6.3 cm3 (4.2, 14.8) or 80% (38, 2008), p = 0.043. In the 4 remaining cases, where no primary was identified, the GTVp + n increased by a median 7.8 cm3 (0, 28.1) or 32% (0,75), p = 0.109. Case 14 was an unusual situation in which a contralateral node was identified by PET. If this is removed from the analysis of the ‘No primary identified’ group the median increase in GTVp + n is 0.2 cm3 (0, 15.3) or 4% (0, 60).
The effect of PET/CT on the CTV
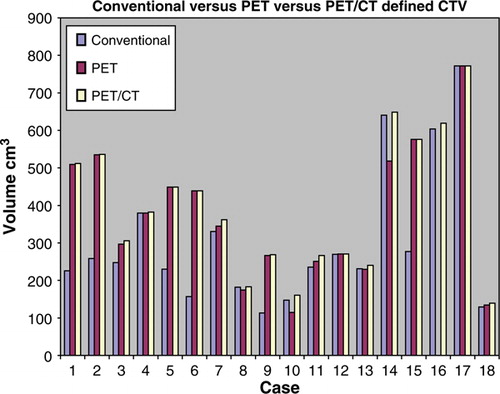
The CTV was defined as shown in . The CTV increased by a median 23 cm3 (8.1, 218.7) or 95% (1,107). summarises these changes. In the nine cases of known primary the CTV increased by a median of 10.1 cm3 (1.3, 30.6) or 4% (0,13), p = 0.012. Case 6 changed by 108% as the CTV grew to include bilateral neck due the GTV coming to midline with the PET/CT defined volumes. In the nine cases of unknown primary, the CTVs outlined were changed following PET/CT in the 5 cases where the primary was identified and in case 14 where a contralateral node was identified. The CTV in all of these 9 cases was increased by a median 155.4 cm3 (2.7, 281.7), p = 0.008. This is a median increase of 95% of the conventionally defined volume. In the five cases where the primary was identified the CTV increased by a median 277.6 cm3 (58.2, 286.3), p = 0.043. This is a median increase of 127%. In the remaining 4 cases, the CTV increased by a median 16.4 cm3 (0.2, 218.7), p = 0.068. This is a median increase of 6%. However if case 14 is taken out of the calculations, the median increase is 1.3 cm3 (0.2, 31.4) or 2%.
Figure 4. Histogram of Clinical Tumour Volume (CTV) defined by Conventional, PET only and PET/CT images
Interestingly, the contralateral node identified by PET/CT in case 14 was not confirmed by histopathology and should be considered a false positive that would have resulted in unnecessary irradiation of the contralateral neck.
The effect of PET/CT on the PTV
The PTV was increased by a median 27.65 cm3 (7.1, 349.8) or 7% (1,101). In the 9 cases of known primary the median increase was 13.3 cm3 (1.7, 41.6) or 5% (0, 12), p = 0.012. In the 9 cases of unknown primary, the overall median increase in the PTV was 276 cm3 (1.7, 393.9) or 101% (0, 134), p = 0.008. In the 5 cases where the primary was identified, this gave a median increase of 393.9 cm3 (91.6, 466.8) or 126%, p = 0.043. In the remaining 4 cases this was 14.5 cm3 (0.5, 349.8), p = 0.068, giving a median increase of 3%. Removing case 14 from the calculations produces a median increase of 1.7 cm3 (0.5, 27.2) or 0%.
Discussion
This study has shown that fused 18FDG-PET/CT images produce different target volumes to those produced using conventional methods. The GTV of the primary tumour was statistically significantly increased by the addition of functional data in the form of PET to the anatomical data of the CT. The PET/CT data allowed easier definition of the primary tumours which were often difficult to visualise on CT alone, even with information from clinical examination and other diagnostic imaging. For patients presenting with carcinoma of unknown primary (CUP) in the head and neck region, the treatment policy at The Royal Marsden Hospital after standard work-up is to perform an ipsilateral modified radical neck dissection followed by hemi-neck irradiation. Therefore, identification of a primary tumour has a significant impact on patient management, and especially for definition of target volumes for radiotherapy planning.
PET/CT identified primary tumours in 5 of 9 cases presenting as unknown primaries, or 56%. This concurs with published data of 57% (12/21) Citation[7] and relates favourably to historical series reporting the impact of 18FDG-PET alone in the search for an unknown primary tumour Citation[8–12]. There are difficulties in drawing comparisons with historical series, because ‘standard’ diagnostic work-up varies widely. Also as PET/CT has become increasingly available, patients are more likely to be referred for a scan at an earlier stage in the investigative pathway. For example, patients may undergo PET/CT before EUA so that biopsies can be directed to areas of FDG uptake. Therefore, it could be argued that had all our 9 patients undergone tonsillectomy those tumours highlighted by FDG uptake in the tonsillar fossa, may have been found.
There are few published accounts of radiotherapy planning studies in HNC using PET. Ciernik Citation[2] took 39 patients with mixed primaries (12 with HNC) and compared target volumes and inter-observer variability when localising using CT alone compared to 18FDG-PET /CT (software-fused). They reported that the gross tumour volume (GTV) changed in 32% with the inclusion of PET data and the mean planning target volume (PTV) change was 20%. Nishioka Citation[13] looked at image fusion between 18FDG-PET and MRI/CT for RTP in 21 patients with HNC. They concluded that the fusion was useful in GTV and CTV determination and enabled sparing of normal tissues. Paulino reported differences in PET-defined GTV compared to CT-defined GTV in 40 patients with HNC that would have resulted in underdosing of PET-defined target volumes if treatment was based on the CT GTV Citation[14].
There are limitations to this study. The CT component of the co-registered images was used for the conventional target definition. As these scans were not contrast enhanced according to the diagnostic protocol. This may have lead to an underestimate of the conventional gross tumour volumes. Immobilisation in this study, where diagnostic scans were used retrospectively, would be inadequate for treatment delivery. Future studies at our institution aim to develop the PET/CT as a treatment planning procedure by scanning the patient in the radiotherapy immobilisation shell.
18FDG-PET/CT has improved the specificity and accuracy of 18FDG-PET alone by providing anatomical correlation for areas of 18FDG uptake. A sensitivity of 91–98% and specificity of 92–93%, an accuracy of 94–96% and a reduction in equivocal lesions by 53% has been reported in diagnosis and staging of HNC using 18FDG-PET/CT Citation[1], Citation[3]. Despite these figures, the exact pathological translation of the 18FDG uptake remains unclear. The combined primary and nodal GTV of all eighteen cases was statistically significantly larger when defined by PET/CT compared to conventional definition. This was due mainly to the five cases where a primary tumour was identified but also because the PET- and conventionally-defined volumes were not the same. As yet, there are insufficient data to allow confidence in editing out PET negative areas from standard radiotherapy target volumes. However, we believe that there are sufficient data to justify the extension of planning volumes to encompass PET positive volumes that lie outside conventionally-defined targets Citation[2], Citation[15–17]. What the required margin around 18FDG avid areas to account for microscopic disease is unknown. Currently radiation treatment planning with CT is still considered the standard of care and there is concern that treatment planning volumes based solely on PET could potentially underestimate the extent of disease. Therefore, we used the composite volumes of both the conventionally-defined and the PET-defined targets. Our results suggest that the use of PET/CT will reduce geographical miss of disease and help to reduce local recurrence rates. We applied the same margin of 1cm to the PET-defined GTV as used with the conventional methods. Neither the optimal margin nor the optimal threshold level of PET-defined target volume for the CTV have been defined.
Published methods of ‘edge-definition’ have varied. Some investigators have chosen to define the PET GTV threshold or cut off as a percentage of the maximum signal intensity Citation[2], Citation[3], Citation[18], Citation[19]. Some have not defined the levels used Citation[20], Citation[21]. Others have used absolute SUVs and auto-contoured all areas above a certain threshold or ratio of background signal Citation[22]. This leads to inconsistent and non-standardisable volumes that are difficult to reproduce. Daisne et al. reported a method for automatic volume segmentation of PET images based on a relationship between source-to-background ratio and the iso-activity level, validated using phantom measurements. However, it was dependent on the image reconstruction algorithm and for smaller lesions (<2 ml) the target volume was overestimated by up to 67%. It was also dependent on the camera used and could not address the problem of complex tumour shapes Citation[23]. Daisne et al. took their work forward and compared volumes defined by their segmentation method with the gold standard pathological specimen in patients with HNC. This showed better correlation than with CT and MRI defined volumes but still an overestimation of pathologically defined volume by 46% Citation[24]. The PET GTVs in this study were defined using a 50% threshold of the local maximum intensity signal given by the planning system software. This is not an absolute threshold of the SUV (Standardised Uptake Value) but of the local activity within the PET voxels. The 50% level was chosen for edge definition in this study following a review of the literature suggested this was the most commonly applied method at the time of starting the study.
The CT GTV in this study was defined by both a radiation oncologist (KN) and a head and neck radiologist (BS). Therefore, the targets were identified jointly as in routine practice but inter-observer variation of GTV definition was not investigated in this study.
The clinical impact of significantly enlarged target volumes needs prospective investigation. Our results would suggest that with decreased geographical miss of disease, rates of tumour control should improve. However the constraints imposed by the critical structures may lessen this impact as intuitively larger target volumes inevitably lead to closer approximation to organs at risk. This may require more complex treatment plans and accurate setup, all of which increase the demands on the radiotherapy planning and treatment units. Treating larger volumes may also limit the use of shorter fractionation regimens.
Conclusion
The addition of functional imaging to conventional means of radiotherapy target definition in HNC can have an effect in several ways. The GTV may not be clearly identifiable with conventional methods and PET/CT can improve this. The GTV itself can be changed in different ways. 18FDG-PET can reveal disease lying outside the conventional target volume, either extending a known area or highlighting a previously unknown area of disease. These changes in GTV lead to changes in the CTV and PTV which may avoid geographical miss. Future studies will shed further light on the effect of such alterations in planning target volume definition on tumour control.
18FDG-PET can also show areas within the conventional target volume that are hypermetabolic which may be possible biological target volumes for dose escalation studies in the future.
We would certainly recommend that all cases presenting as CUP should have a PET/CT prior to radiotherapy planning. If a primary site is then identified this should be biopsied and if pathological confirmation is obtained, the PET/CT images should be used for the planning of radiotherapy target volumes. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Branstetter BFt, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: Is PET/CT more accurate than PET or CT alone?. Radiology 2005; 235: 580–6
- Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): A feasibility study. Int J Radiat Oncol Biol Phys 2003; 57: 853–63
- Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: Clinical usefulness and accuracy of PET/CT image fusion. Radiology 2004; 231: 65–72
- International Commission on Radiation Units and Measurements ICRU Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50), ICRU, Bethesda, MD(1999).
- Gregoire V, Haustermans K, Geets X, Roels S, Lonneux M. PET-based treatment planning in radiotherapy: A New Standard?. J Nucl Med 2007; 48(Suppl 1)S68–S77
- Humphreys M, Guerrero Urbano MT, Mubata C, Miles E, Harrington KJ, Bidmead M, et al. Assessment of a customised immobilisation system for head and neck IMRT using electronic portal imaging. Radiother Oncol 2005; 77: 39–44
- Freudenberg LS, Fischer M, Antoch G, Jentzen W, Gutzeit A, Rosenbaum SJ, et al. Dual modality of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in patients with cervical carcinoma of unknown primary. Med Princ Pract 2005; 14: 155–60
- Wong WL, Saunders M. The impact of FDG PET on the management of occult primary head and neck tumours. Clin Oncol (R Coll Radiol) 2003; 15: 461–6
- Gutzeit A, Antoch G, Kuhl H, Egelhof T, Fischer M, Hauth E, et al. Unknown primary tumors: Detection with dual-modality PET/CT–initial experience. Radiology 2005; 234: 227–34
- Kresnik E, Mikosch P, Gallowitsch HJ, Kogler D, Wiesser S, Heinisch M, et al. Evaluation of head and neck cancer with 18F-FDG PET: A comparison with conventional methods. Eur J Nucl Med 2001; 28: 816–21
- Greven KM, Keyes JW, Jr, Williams DW, 3rd, McGuirt WF, Joyce WT, 3rd. Occult primary tumors of the head and neck: Lack of benefit from positron emission tomography imaging with 2-[F-18]fluoro-2-deoxy-D-glucose. Cancer 1999; 86: 114–8
- OS AA, Fischbein NJ, Caputo GR, Kaplan MJ, Price DC, Singer MI, et al. Metastatic head and neck cancer: Role and usefulness of FDG PET in locating occult primary tumors. Radiology 1999; 210: 177–81
- Nishioka T, Shiga T, Shirato H, Tsukamoto E, Tsuchiya K, Kato T, et al. Image fusion between 18FDG-PET and MRI/CT for radiotherapy planning of oropharyngeal and nasopharyngeal carcinomas. Int J Radiat Oncol Biol Phys 2002; 53: 1051–7
- Paulino AC, Thorstad WL, Fox T. Role of fusion in radiotherapy treatment planning. Semin Nucl Med 2003; 33: 238–43
- Schwartz DL, Ford E, Meyer J, Rajendran J, Lewellen B, Yueh B, et al. Co-registered FDG-PET/CT imaging for staging and IMRT treatment planning for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2003; 57(2 Suppl)S156–S157
- Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck 2005; 27: 494–502
- Scarfone C, Lavely WC, Cmelak AJ, Delbeke D, Martin WH, Billheimer D, et al. Prospective feasibility trial of radiotherapy target definition for head and neck cancer using 3-dimensional PET and CT imaging. J Nucl Med 2004; 45: 543–52
- Mah K, Caldwell CB, Ung YC, Danjoux CE, Balogh JM, Ganguli SN, et al. The impact of (18)FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small-cell lung carcinoma: A prospective study. Int J Radiat Oncol Biol Phys 2002; 52: 339–50
- Nestle U, Walter K, Schmidt S, Licht N, Nieder C, Motaref B, et al. 18F-deoxyglucose positron emission tomography (FDG-PET) for the planning of radiotherapy in lung cancer: High impact in patients with atelectasis. Int J Radiat Oncol Biol Phys 1999; 44: 593–7
- Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004; 59: 78–86
- Kalff V, Hicks RJ, MacManus MP, Binns DS, McKenzie AF, Ware RE, et al. Clinical impact of (18)F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: A prospective study. J Clin Oncol 2001; 19: 111–8
- Vanuytsel LJ, Vansteenkiste JF, Stroobants SG, De Leyn PR, De Wever W, Verbeken EK, et al. The impact of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) lymph node staging on the radiation treatment volumes in patients with non-small cell lung cancer. Radiother Oncol 2000; 55: 317–24
- Daisne JF, Sibomana M, Bol A, Cosnard G, Lonneux M, Gregoire V. Evaluation of a multimodality image (CT, MRI and PET) coregistration procedure on phantom and head and neck cancer patients: Accuracy, reproducibility and consistency. Radiother Oncol 2003; 69: 237–45
- Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: Comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004; 233: 93–100